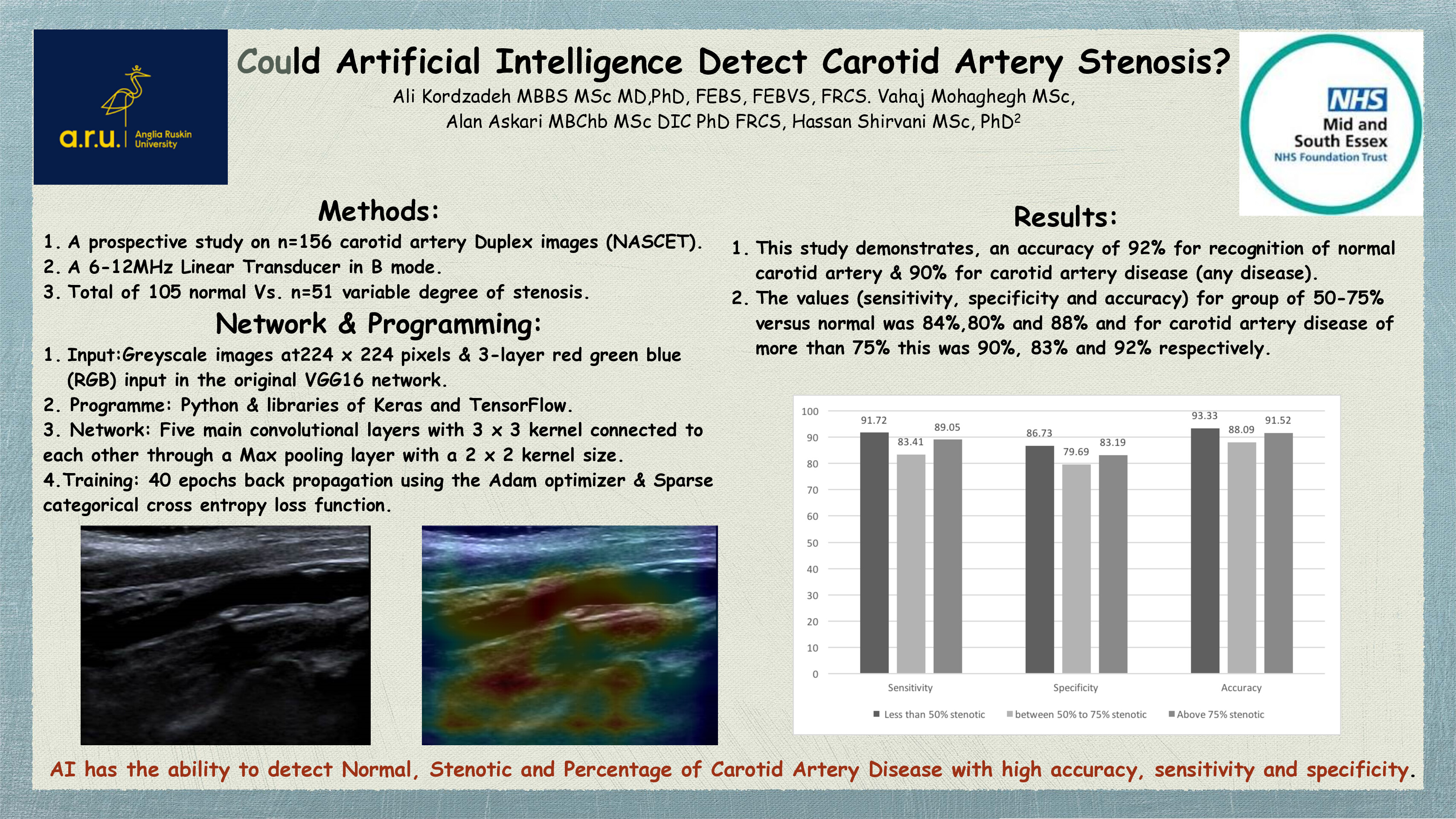
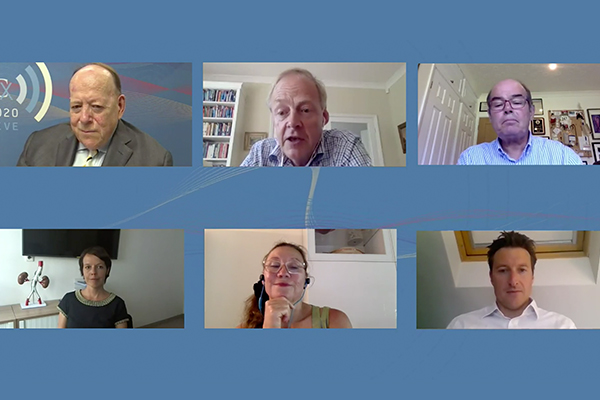
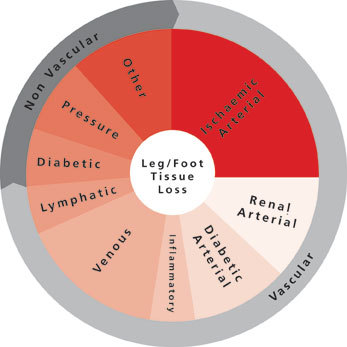
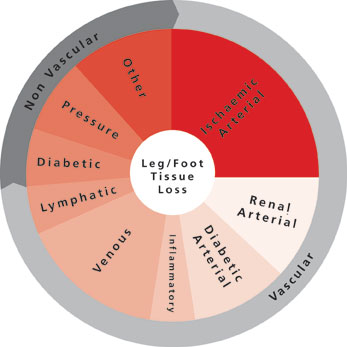
CX 2024: Significant involvement from vascular trainees in this year’s vascular trauma programme
honorapamplin2024-04-15T15:41:56+01:00The vascular trauma programme at […]
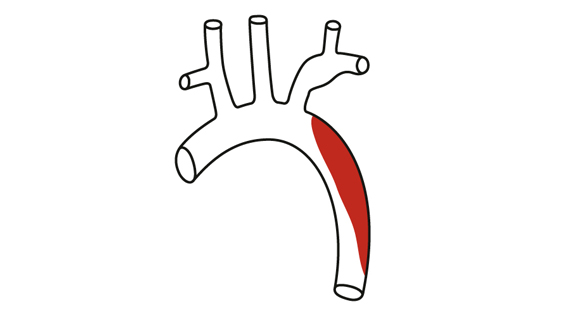
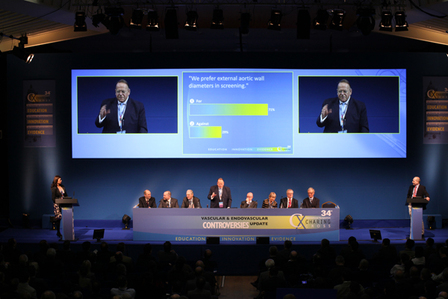
CX 2024: Don’t miss a series of great aortic debates by our world class faculty
honorapamplin2024-04-12T18:43:02+01:00
CX 2024: An outstanding faculty will tee up timely debates to enhance vascular education
Dittmar Böckler (Heidelberg, Germany), one of co-chairs of CX 2024, rings the CX bell, and calls out the […]
CX 2024: Delivers on the complex and everyday issues in Vascular Access and Renal Interventions
honorapamplin2024-04-10T15:48:58+01:00[…]
CX 2024: A dynamic force shaping the global vascular community
honorapamplin2024-04-05T10:54:02+01:00The CX 2024 venous programme has “a lot to look forward to”
Register today to secure your place at the CX 2024 Venous & Lymphatic Controversies sessions taking place […]
CX 2024: New data and heated debates set to spark controversy in CX peripheral arterial programme
honorapamplin2024-04-03T10:19:03+01:00Andrew Holden (Auckland, New Zealand) CX Co-Chair and member of the Peripheral Executive Board highlights the top sessions coming in the CX Peripheral Controversies Update.
Register today to secure your place […]
CX 2024: Aortic Programme forefronts cutting-edge edited cases “on film” and multiple top-notch debates
[email protected]2024-04-02T18:39:06+01:00Tilo Kölbel (vascular surgeon from Hamburg, Germany, and a member of the Executive Board for the Aortic Programme) makes the case for moving images that will “easily […]
CX 2024: Brand new data on cyanoacrylate glue, bioprosthetic valves and intensive pulmonary embolism treatments
[email protected]2024-03-26T14:31:01+00:00Manj Gohel (Cambridge, UK) and member of the Venous Executive Board parachutes straight into the CX Venous and Lymphatics Controversies Programme to highlight the VenaSeal SPECTRUM programme, SAVVE trial and SYNCHRONOUS trial, which will all present results for the very first time to an international audience at the Charing Cross International Symposium 2024 […]
CX 2024: Get a ringside seat for the big BASIL-3 data reveal
[email protected]2024-03-14T11:40:59+00:00No more freestyling, reset your endovascular CLTI practice with new Level 1 evidence
Andrew Bradbury (Birmingham, UK) and the BASIL-3 team of triallists will be presenting, for the very first time, the results of this long-awaited, only fully publicly funded randomised controlled trial (RCT) at CX 2024 (23–25 April, London, UK).
45 Year Legacy of CX Vascular Education Continues
[email protected]2023-10-10T12:59:22+01:00Three newly appointed Charing Cross International Symposium (CX) Co-chairs will ensure Roger Greenhalgh’s inspiring legacy of vascular education continues at CX 2024 with the 46th CX Symposium. Dittmar Böckler (Heidelberg, Germany), Andrew Holden (Auckland, New Zealand) and Erin Murphy (Charlotte, United States) speak about CX founder Roger Greenhalgh’s lasting legacy and the honour of leading […]
CX 2024: Chairman’s Address
[email protected]2023-09-21T14:39:04+01:00| Filmed in the newly completed CX Studio, CX Chair Professor Roger Greenhalgh (London, UK), outlines the plans for CX 2024. Now in its 46th year, the Charing Cross Symposium continues to champion world-class education, innovation and evidence and opens its doors to the global vascular and endovascular community.
Don’t miss […] |
CX 2024: The Delegate Perspective
[email protected]2023-09-06T11:03:17+01:00What to Expect In-Person @ CX 2024
I attend CX to network with international colleagues in the field of vascular surgery. The best part of CX was seeing my colleagues again in-person after the pandemic years.
The best thing for me about Charing Cross was the opportunity to deal hands-on with new technologies.
The IT […]
How to Write a Successful Abstract
[email protected]2023-10-10T16:40:58+01:00Abstract submissions for CX 2024 are now open! We are inviting senior and trainee clinicians working in the vascular and endovascular field to submit abstracts to be considered for in-person presentations and electronic posters in the CX 2024 Vascular & Endovascular Controversies Update, in-person and virtual from London, UK, 23-25 April 2024 (Tuesday to […]
CX 2023 Abstract Winners
[email protected]2023-10-10T16:53:18+01:00The CX Executive and Abstract Boards would like to congratulate the senior and trainee clinicians who presented their abstracts at the Charing Cross Symposium 2023. In total over 50 abstracts were presented across the main programme and additional activities coving abdominal, peripheral, aortic arch, venous & lymphatic, vascular trauma, acute stroke and vascular access topics.
The […]
CX 2023 Abstract Poster Winners
[email protected]2023-05-09T14:00:12+01:00CX 2023 received over 350 abstract submissions and saw 50+ live presentations during the event. The presented abstracts were scored by an expert panel of moderators. The selected abstract sessions featured poster presentations from researchers from across the globe.
Abstract Poster Winner: Ali Kordzadeh
The top scoring abstract poster […]
CX 2023 Hurting Leg Competition Winners
[email protected]2023-05-05T15:13:46+01:00We are happy to announce that the winning infomercial and infographics for the Hurting Leg Competition have been chosen!
The winners were selected via online public vote during the Rouleaux Club’s programme session on Thursday 27 April 2023. To relive this fantastic moment, you can view the session in full on-demand (you must be registered for […]
CX 2023: Get the most from your Charing Cross experience!
[email protected]2023-04-18T11:41:20+01:00Watch this video to discover how to get the most from your CX experience – for both in-person and virtual delegates.
This video demonstrates how to use the mycx app / mycxonline web platform.
Learn how to;
– Access the full event programme
– Watch live content
– Participate in live discussion
– Create your own daily agenda
– Watch […]
CX 2023: 45 Years of Looking Forward
[email protected]2023-04-14T10:56:48+01:00
CX 2023: 45 Years of Looking Forward
- 1978: Founded in 1978, the Charing Cross Symposium has gone on to become the leading global vascular symposium.
First held at the Drew Lecture Theatre of the Charing cross Hospital Medical School, CX provides first-class Education, Innovation and Evidence presented by world-leading experts.
- 1987: Dr. Michael DeBakey, vascular pioneer, […]
CX 2023: Reaching a Peripheral Consensus with an Expert Faculty
[email protected]2023-04-12T12:24:11+01:00We spoke with Marianne Brodmann (Graz, Austria) who kindly shared her highlights of the upcoming Charing Cross 2023 programme. Dr. Brodmann also speaks on a number of interesting topics including; the value of a transradial approach for vascular disease interventions, predictors of drug-coated balloon efficacy in femoropopliteal intervention, the BEST CLI trial and the importance […]
CX 2023: Innovation and Audience Interaction
[email protected]2023-04-03T16:54:53+01:00Professor Andrew Holden (Auckland, New Zealand) is really excited about the 18 podium first presentations showcasing new data at CX 2023 and uniquely, the interaction between a world-class faculty and expert audience.
Register now to attend CX 2023! Take a look at the upcoming podium first highlights below.
| CX 2023 – PODIUM FIRST: |
CX 2023: Challenging the Vascular Status Quo
[email protected]2023-03-31T15:19:32+01:00
In this conversation between CX chairman Roger Greenhalgh and Professor of Vascular Surgery, Maarit Venermo (Helsinki, Finland), we discuss the importance of challenging the status quo in vascular and endovascular surgery.
Professor Venermo discusses challenging the status quo in superficial femoral artery management, non-invasive methods and her thoughts on aneurysms in the carotid artery “I’ve heard some […]
CX 2023: Reaching Venous Consensus with a World-Class Faculty
[email protected]2023-03-21T15:55:58+00:00“I think the Charing Cross programme has worked very hard to make a comprehensive and up-to-date consensus on what we have available to us now, what we know now and where we are going.”
This week we sat down with Executive Board member and vascular surgeon Erin Murphy (Charlotte, United States) to talk […]
CX 2023: Aortic Techniques & Technologies Highlights
[email protected]2023-03-06T16:46:42+00:00Start planning your CX 2023 itinerary today! We sat down with CX Executive Board member, Tilo Kölbel (Hamburg, Germany), to discuss the upcoming presentations in the Aortic techniques & technologies sessions. Register for the CX 2023 Consensus Update today!
Tilo Kölbel’s Highlights:
- Edited Case: Guo’s aortic arch reconstruction: A prospective, multiple center study about the safety and efficacy of […]
CX 2023: Andrew Bradbury discusses BASIL-2 trial
[email protected]2023-03-01T12:20:34+00:00“I am really looking forward to seeing everyone at CX 2023 and it is going to be a great meeting. It is a pleasure and a privilege for us to be part of it and have the opportunity to present our trial data for the first time.”
Data and discussion on revascularisation treatment […]
CX 2023: Rouleaux Club’s Hurting Leg Competition
[email protected]2023-02-06T10:23:39+00:00The Rouleaux Club, in association with Charing Cross International Symposium 2023 and BIBA Medical Ltd, are holding a competition to create an infographic and/or infomercial intended to educate members of the public in Chronic Limb Threatening Ischaemia (CLTI) in a competition entitled “The Hurting Leg”. The competition will hopefully “encourage patients to present early to their GPs with […]
CX 2023: Inspiring the Next Generation of Vascular Specialists with Sophie Renton
[email protected]2023-02-03T16:35:46+00:00“It is absolutely key that we enthuse the young and that we show them what we can do in order to be interested in joining our professions.”
CX is offering more opportunities and benefits for trainees, fellows, early career surgeons and physicians than ever before. This week we sat down with Sophie Renton (London, UK), a consultant […]
CX 2023: Reaching Peripheral Arterial Consensus with a World-Class Faculty
[email protected]2023-01-04T11:18:41+00:00Endovascular and Interventional Radiology Consultant Bella Huasen (Preston, United Kingdom) stopped by the CX studio to chat about the upcoming Charing Cross Symposium in April 2023.
Like all of us, she is looking forward to CX – to networking, debating and reaching consensus on peripheral arterial topics. We will be welcoming over 2,500 delegates, virtually and […]
The Top Cardiovascular News Stories of 2022!
[email protected]2023-01-02T03:57:22+00:00What a year! We are taking a look back at the most viewed news stories. Check out these top stories and videos and make sure you haven’t missed any of 2022’s most-viewed news!
Remember – you can still claim up to 24 reciprocal EU/US CME credits for viewing CX 2022 and CX […]
Seasons Greetings and a Happy New year for 2023
cxsymposium2022-12-20T09:42:39+00:00
As we reach the end of another busy year, the BIBA Medical team – organising CX 2023, publishing our newspaper and delivering customer and marketing insights – wanted to take this opportunity […]
What to Expect at Charing Cross 2023
[email protected]2023-01-02T04:01:36+00:00As the year comes to a close, we are looking forward to 2023 and what is in store for CX attendees in April! There are so many fantastic reasons to attend CX in-person or online, see you in 2023!
What to Expect at CX 2023:
- First-time data releases
- Debates
- Masterclasses
- Workshops
- CME points
- Exclusive networking opportunities
[…]
CX 2023: Reaching Renal Interventional and Vascular Access Consensus with a World-Class Faculty
[email protected]2022-12-09T17:21:06+00:00This year, we aim to reach consensus on renal interventional and vascular access issues. With the help of a world-class faculty, we will be leading a one day vascular access masterclass and workshop in London, which is the largest in Europe. We spoke with CX executive board members, Nick Inston (Birmingham, UK) and Kate Steiner […]
CX 2023: Reaching Aortic Consensus with a World-Class Faculty!
[email protected]2022-12-01T17:02:54+00:00This year we expect over 2,500 in-person and 1,000 virtual participating vascular specialists to join us. In previous years we have hosted over 6,000 registrants virtually from over 120 countries due to the COVID-19 pandemic and are very excited to be back in London 25-27 April 2023. To make CX as accessible as possible, registrants will also have […]
CX 2023: Reaching Venous and Lymphatic Consensus with a World-Class Faculty
[email protected]2022-12-15T12:26:15+00:00CX 2023 is seeking to reach venous and lymphatic consensus on topics related to the superficial, deep and pelvic veins as well as venous ulcers and lymphatics. Reaching consensus this year will round out our three-year cycle of controversies, challenges and consensus. Topics will be addressed by the world-class CX faculty alongside […]
Edited case in the spotlight: Long-term clinical and patient focused outcomes for no-hope CLTI patients, Miguel Montero-Baker (Houston, United States)
Adam Pearce2022-08-09T12:18:13+01:00
Dr Miguel Montero-Baker (Houston, United States) stresses that the current disease state of peripheral arterial disease (PAD) is leading to more complex below the ankle calcification patterns, specifically in renal failure patients. Within the edited case Montero-Baker champions transcatheter arterialisation of deep veins (TADV) with the LimFlow device, stating it is a procedure […]
Edited case in the spotlight: In stent restenosis and occlusion – an atherectomy plus DCB strategy, Martin Andrassy (Bruchsal, Germany)
Adam Pearce2022-08-09T12:15:46+01:00
Professor Martin Andrassy (Bruchsal, Germany) advocates that Jetstream Atherectomy can be used in all types of lesions effectively and safely. Andrassy notes that embolism occurs more often in in-stent restenosis (ISR) than in long de novo lesions, but the rare […]
CX 2022: Watch the pivotal discussions on-demand!
Adam Pearce2022-08-09T11:50:14+01:00Benefit from up to 24 reciprocal EU/US CME credits
Watch the global vascular field unite on our dedicated CX 2022 on-demand channel and re-live the decisive moments […]
CX 2022: Choose to attend in-person or virtually
Adam Pearce2022-08-09T11:57:26+01:00We warmly invite our international friends and those unable to attend in-person to London to join us virtually at CX 2022. The world-class programme will be livestreamed and available on-demand, so you can watch whenever you’re ready – even after the event.
Virtual audience members can submit questions and join in on polling […]
CX2022: CX Innovation Showcase
Adam Pearce2022-08-09T11:48:24+01:00
Dear friends,
The Charing Cross (CX) Symposium is about delivering world class vascular education, considering the evidence and showcasing innovation. The CX Innovation Showcase is dedicated to highlighting innovation, what it takes for a physician-inventor to succeed and for innovative ideas in the vascular and endovascular arena to […]
CX 2022: Dr Rob Hinchliffe on Hurting Leg Challenges
Adam Pearce2022-08-09T11:52:41+01:00“An interdisciplinary problem”
CX 2022 will highlight Hurting Leg Challenges, addressing unbalanced amputation rates based on geography, distilling the underlying causes of “hurting legs” and getting patients into the right hands. Be a part of it – register your place and explore the programme online.
CX Executive […]
CX 2022: What to expect
Adam Pearce2022-08-09T11:55:32+01:00CX 2022 will unite the global vascular and endovascular community in-person, at the Hilton London Metropole, UK, and virtually (April 26–28, 2022). The most pressing vascular and endovascular challenges will be addressed by global thought leaders alongside audience participation, discussion and polling. Be a part of it – register your place
CX 2022: A conversation with Dr Erin Murphy
Adam Pearce2022-08-09T12:28:19+01:00
CX 2022 will highlight Venous and Lymphatic Challenges, focusing on appropriate care conundrums to venous challenges in relation to the “hurting leg”. Challenges related to the superficial, deep and pelvic veins, as well as venous wounds, will be addressed by global thought leaders alongside audience participation, polling and discussion. Be a part […]
CX returns to in-person format once more in the London spring
Adam Pearce2022-03-02T13:04:57+00:00CX 2022: Haulon, Mastracci and Kölbel on Aortic Challenges
Adam Pearce2022-01-12T11:22:00+00:00Global thought leaders speak to CX, the first opportunity of the year to unite the vascular and endovascular field, face-to-face, about the challenges and opportunities at the crux of the aortic field. You can register with the early bird rates now.
Stéphan Haulon (Paris, France), highlights the […]
CX 2022: Nicholas Inston on Vascular Access Challenges
Adam Pearce2021-12-15T11:24:26+00:00Nicholas Inston (Birmingham, UK) talks to CX about the challenges in creation, maintenance and salvage of vascular access in patients with end stage renal disease. Inston highlights the […]
CX Aortic Vienna 2021: Watch the key pivotal discussions on-demand! Day 2 Highlights
cxsymposium2021-10-20T16:50:59+01:00CX Aortic Vienna 2021: Watch the key pivotal discussions on-demand!
Adam Pearce2021-10-14T15:59:55+01:00Benefit from up to 15 reciprocal EU/US CME credits
Watch the cardiac, vascular and […]
CX 2021: Thoracic Aortic Highlights
Adam Pearce2021-05-13T15:21:07+01:00There is still time to register for CX 2021 on-demand and catch up on the first-class Education, Innovation, and Evidence presented by world-leading experts.
Day 3 of Livestream […]
CX 2021: Deep Venous Highlights
Adam Pearce2021-05-13T15:19:43+01:00There is still time to register for CX 2021 on-demand and catch up on the first-class Education, Innovation, and Evidence presented by world-leading experts.
History was made on Livestream 2 during the Deep Venous Controversies session when Joseph […]
CX 2021: Vascular Access Highlights
Adam Pearce2021-05-06T18:00:51+01:00Watch history unfold on our dedicated CX 2021 on-demand channel and relive the decisive debates of the event, whilst also benefiting from up to 16 reciprocal EU/US CME credits.
CX is privileged to be the platform to host these high-impact findings and invite you to watch on-demand to […]
CX 2021: Peripheral Arterial Proximal Highlights
Adam Pearce2021-05-06T18:00:08+01:00Watch history unfold on our dedicated CX 2021 on-demand channel and relive the decisive debates of the event, whilst also benefiting from up to 16 reciprocal EU/US CME credits.
Highlighted from Day 2 was the long-awaited call to change agency recommendations regarding paclitaxel use in peripheral interventions, spearheaded […]
CX 2021: Venous and Lymphatic Highlights
Anthony Strzalek2021-04-29T20:09:24+01:00CX 2021 has finished but that does not mean you have missed your opportunity to view all the high-quality content that was on display. All our sessions, from both Livestream 1 and Livestream 2, are available on-demand on our dedicated CX 2021 channel.
To get access to the entire content from […]
CX 2021: Abdominal Aortic Highlights
Anthony Strzalek2021-04-29T20:06:21+01:00CX 2021 has finished but that does not mean you have missed your opportunity to view all the high-quality content that was on display. All our sessions, from both Livestream 1 and Livestream 2, are available on-demand on our dedicated CX 2021 channel.
To get access to the entire content from […]
Experts go head-to-head in Acute Stroke session
Anthony Strzalek2021-04-11T10:54:56+01:00Barbara Rantner (Munich, Germany; CX Executive Board Member) discusses some of the upcoming highlights from the session including an array of debates which are outlined below and which Rantner notes “really matter to the global vascular community”.
You can register for CX 2021 here.
Vascular Trauma Controversies at CX 2021
Anthony Strzalek2021-04-01T18:09:16+01:00Christopher Aylwin (UK) talks about resuscitative endovascular balloon occlusion of the aorta (REBOA), which he notes will likely be the biggest controversial topic in this year’s Vascular […]
CX 2021: Vascular Access programme targets impact of high-flow arteriovenous fistulas
Anthony Strzalek2021-03-26T14:09:26+00:00“International practice differences exist in cannulation and dialysis provision with some approaches aiming for fistulas to suit the logistics of dialysis, rather than best long-term outcomes for the fistula and the patient”
The CX 2021 Vascular Access Controversies Programme is designed to showcase the latest advances and disputes […]
‘Unacceptably high’ major amputation rates and the effects of COVID on the agenda in Hurting Leg session
Anthony Strzalek2021-03-25T09:33:07+00:00William Jeffcoate (UK) discusses his upcoming presentation at the Hurting Leg session at CX 2021, titled The impact of socio-economic and clinical variables on the incidence of major amputation in people with diabetic foot ulcers.
The session, which will take place on Thursday 22 April, will shine a […]
World-class Faculty lined up to tackle Superficial Venous & Lymphatic Controversies
Anthony Strzalek2021-03-05T15:24:48+00:00Lowell Kabnick (USA) talks about what he is looking forward to in the upcoming Venous & Lymphatic Controversies (Superficial) session at CX 2021. He also discusses his own presentation, Is there evidence to justify an aggressive strategy for the ablation of incompetent perforators?. This controversial topic is “important to the […]
Lively debates will tackle the biggest controversies in Abdominal Aortic session
Anthony Strzalek2021-03-03T14:40:10+00:00Colin Bicknell (UK) and Michael Jenkins (UK) will go toe-to-toe in a hotly anticipated debate at CX 2021 titled Standard EVAR can be used in most challenging necks. Bicknell will be arguing for the motion and Jenkins–who is current president of the Vascular Society of Great Britain and Ireland (VSGBI)–will be […]
Paclitaxel controversy and Podium 1st presentations take centre stage in Peripheral session
Anthony Strzalek2021-03-01T09:35:45+00:00Andrew Holden (New Zealand; CX Executive Board Member) discusses some of the main features of the upcoming Peripheral Proximal session at CX 2021 as well as his Podium 1st presentation–Five-year meta-analysis including mortality update with FDA-approved RCTs of paclitaxel devices.
You can register for CX 2021 here. […]
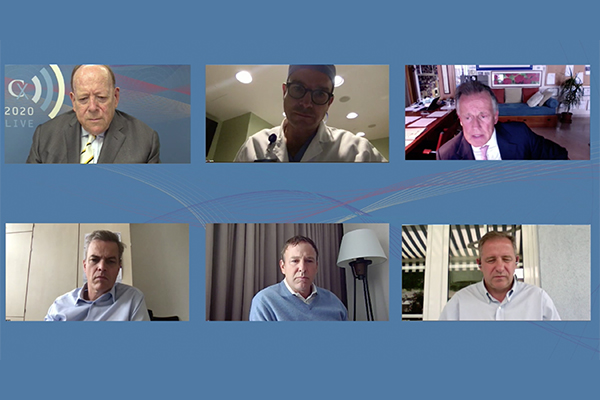
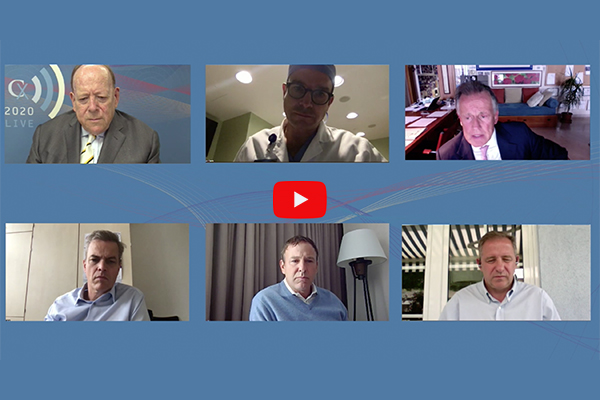
CX 2020 LIVE: Surgeon-modified and custom-made endografts carve their place in juxtarenal aneurysm treatment
Anthony Strzalek2020-06-26T17:23:41+01:00 Clockwise from top left: Roger Greenhalgh (London, UK), Stéphan Haulon (Paris, France), Gustavo Oderich (Rochester, USA), Nikolaos Tsilimparis (Munich, Germany), Bijan Modarai (London, UK) and Said Abisi (London, UK). Click on the image to watch the Juxtarenal Aneursym Consesus session on demand.
Clockwise from top left: Roger Greenhalgh (London, UK), Stéphan Haulon (Paris, France), Gustavo Oderich (Rochester, USA), Nikolaos Tsilimparis (Munich, Germany), Bijan Modarai (London, UK) and Said Abisi (London, UK). Click on the image to watch the Juxtarenal Aneursym Consesus session on demand.
During […]
CX 2020 LIVE: The conversation on challenges in acute stroke continues
dawn2020-06-24T17:31:17+01:00CX 2020 LIVE audience delivers resounding vote in favour of fibre optic visualisation
Anthony Strzalek2020-06-19T17:38:09+01:00CX 2020 LIVE: Overwhelming 91% of specialist audience favours venous stents
dawn2020-06-19T13:35:41+01:00 Roger Greenhalgh (top left), Armando Mansilha (top middle), Manj Gohel (top right), Christopher Cheng (bottom left), Michael Jolly (bottom middle), and Houman Jalaie (bottom right)
Roger Greenhalgh (top left), Armando Mansilha (top middle), Manj Gohel (top right), Christopher Cheng (bottom left), Michael Jolly (bottom middle), and Houman Jalaie (bottom right)
A poll of an enthused audience of venous specialists during the CX 2020 LIVE Deep Venous Disease Consensus […]
CX 2020 LIVE: Podium 1st Session to showcase new technologies and treatments in abdominal and thoracic aorta
Anthony Strzalek2020-06-17T17:56:25+01:00Roberto Chiesa (Milan, Italy) invites you to the CX 2020 LIVE Aortic Podium 1st Session which will feature abdominal aortic and thoracic aortic presentations and discussion from specialists in the field, including Tilo Kölbel, Frank Arko, Eric Verhoeven, Stephan Haulon, Giovanni Torsello and Fabio Verzini.
The session, which begins on […]
CX 2020 LIVE: Vascular Access session showcases new technologies, while debate on place of endovascular fistula creation continues
Anthony Strzalek2020-06-12T16:53:54+01:00CX 2020 LIVE: Deep venous session to examine stent durability and design
Anthony Strzalek2020-06-11T13:20:21+01:00Armando Mansilha (Porto, Portugal), who is also a member of the CX Venous Executive Board, welcomes you to the Deep Venous Disease Consensus session at CX 2020 LIVE. Deep venous disease is a “significant medical problem for patients” and also a “substantial burden for our healthcare systems”, notes Mansilha.
In […]
CX 2020 LIVE: Strong support for relining peripheral arteries and rivaroxaban use for widespread atherosclerosis
Aaron Kudhil2020-06-10T16:28:10+01:00 Roger Greenhalgh (top left), Gunnar Tepe (top right), Jean-Paul de Vries (bottom left), Andrew Holden (bottom middle) and Amer Zanabili (bottom right)
Roger Greenhalgh (top left), Gunnar Tepe (top right), Jean-Paul de Vries (bottom left), Andrew Holden (bottom middle) and Amer Zanabili (bottom right)
CX 2020 LIVE aortic arch discussion highlights importance of underlying pathology and benefit of multidisciplinary team
cxsymposium2020-06-05T17:33:02+01:00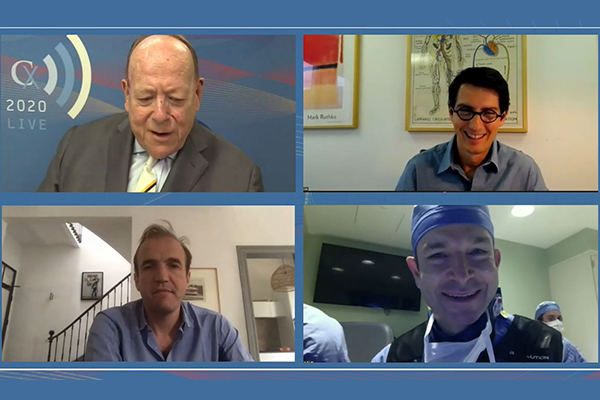 Roger Greenhalgh (top left), Stéphan Haulon (top right), Ludovic Canaud (bottom left), and Gustavo Oderich (bottom right)
Roger Greenhalgh (top left), Stéphan Haulon (top right), Ludovic Canaud (bottom left), and Gustavo Oderich (bottom right)
This week, the CX 2020 LIVE agenda turned to the technically challenging topic of aortic arch interventions. Through presentations, discussion, and polling, the session—chaired by Roger Greenhalgh (London, UK) and […]
CX 2020 LIVE panel considers advances in thoracic care with new developments in TEVAR
Anthony Strzalek2020-06-05T15:07:47+01:00 Ali Azizzadeh (top) chaired the CX 2020 LIVE Industry Symposium, which featured contributions from Hence Verghagen (bottom row left), Richard Gibbs (bottom row left), and Santi Trimarchi (bottom row right)
Ali Azizzadeh (top) chaired the CX 2020 LIVE Industry Symposium, which featured contributions from Hence Verghagen (bottom row left), Richard Gibbs (bottom row left), and Santi Trimarchi (bottom row right)
Benefits of using angulation control in thoracic endovascular aortic repair (TEVAR), an update […]
CX 2020 LIVE gains CME accreditation: Attendees from 79 countries participate live
dawn2020-06-03T18:13:26+01:00CX 2020 LIVE features key registry data on EVAR durability with EXCLUDER Conformable AAA Endoprosthesis
Anthony Strzalek2020-06-02T09:27:26+01:00Data from three global, real-world registries, GREAT, ExCeL and BSET-CLEVAR, evaluating the Excluder Conformable abdominal aortic aneurysm (AAA) Endoprosthesis with Active Control System, were shared online during the CX 2020 LIVE virtual, vascular conference. Broadcast to a global audience at the conclusion of the second […]
Nearly 70% of CX 2020 LIVE global aortic audience finds type II endoleak without sac expansion harmless
cxsymposium2020-06-02T09:04:25+01:00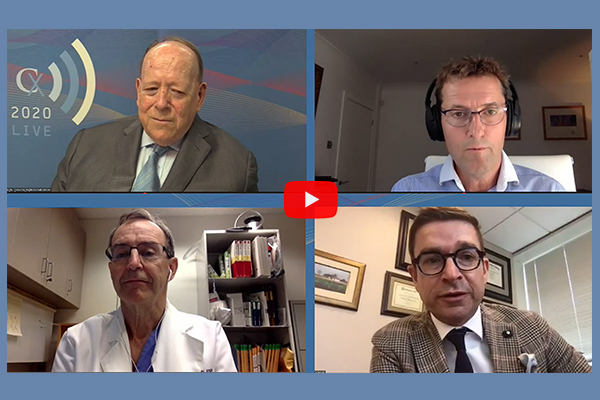 Roger Greenhalgh (top left), Ian Loftus (top right), Lindsay Machan (bottom left), and Gustavo Oderich (bottom right)
Roger Greenhalgh (top left), Ian Loftus (top right), Lindsay Machan (bottom left), and Gustavo Oderich (bottom right)
A global audience tuned into the second session and inaugural aortic offering of CX 2020 LIVE. […]
CX 2020 LIVE pioneers virtual vascular conference format in COVID-19 era
cxsymposium2020-06-02T09:11:44+01:00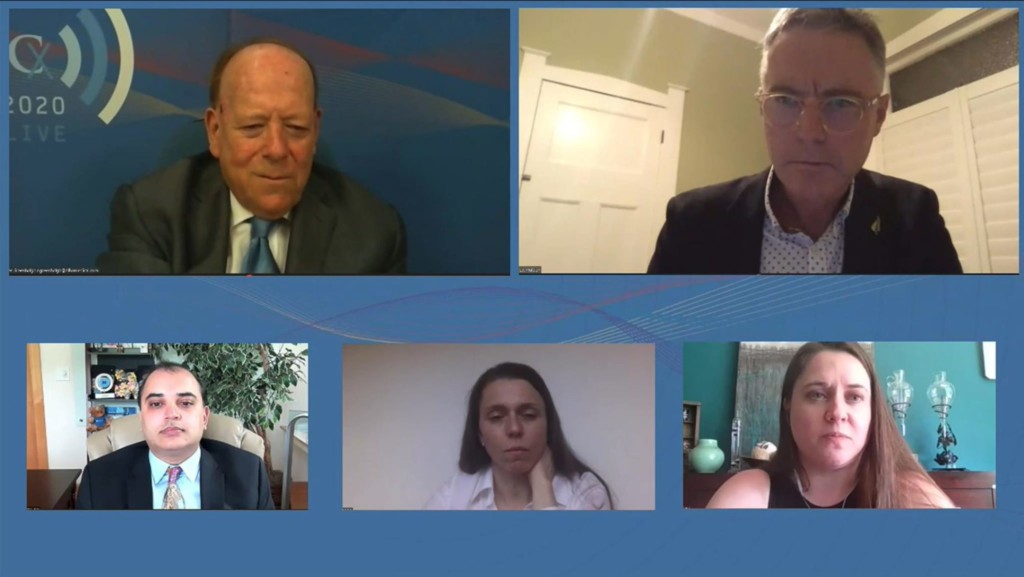 Chair Roger Greenhalgh (top left), moderator Andrew Holden (top right), Sapan Desai (bottom left), Sabine Steiner (bottom middle), and Misti Malone (bottom right)
Chair Roger Greenhalgh (top left), moderator Andrew Holden (top right), Sapan Desai (bottom left), Sabine Steiner (bottom middle), and Misti Malone (bottom right)
CX 2020 LIVE came to life online—despite COVID-19—using state-of-the art broadcast technology to bring together more than 1,000 vascular specialists, […]
Covered endoprostheses gain key role in management of aortoiliac occlusive disease
dawn2020-06-02T08:57:44+01:00 Michele Antonello (top) chairs the discussion, following the Industry Symposium, with Amer Zanabili and Bella Huasen.
Michele Antonello (top) chairs the discussion, following the Industry Symposium, with Amer Zanabili and Bella Huasen.
The first session of CX 2020 LIVE also featured an Industry Symposium titled “Endovascular treatment of complex aortoiliac occlusive disease” (sponsored by Gore). Chaired by Michele Antonello […]
CX 2020 LIVE: Vascular Access education and innovation “imperative” during COVID-19 crisis
Anthony Strzalek2020-05-22T16:58:23+01:00
CX 2020 LIVE will consist of 10 live sessions, which will feature audience participation, and run from 16:00 BST/11:00 EDT/17:00 CEST every Tuesday and Thursday, from 26 May until 25 June. Make sure you register now to participate in the CX 2020 LIVE sessions.
Nicholas Inston (Birmingham, UK), co-chair of the Vascular […]
CX 2020 LIVE to flag latest guidelines and stent durability in superficial and deep venous programmes
Anthony Strzalek2020-05-20T12:57:01+01:00Manj Gohel (Cambridge, UK), who is also a member of the CX Venous Executive Board, welcomes you to CX 2020 LIVE, which will include some of the major highlights from the deep and superficial venous programmes.
A number of recently released “important guidelines” in the superficial venous space will be presented […]
CX 2020 LIVE: Durability of EVAR, sac diameter and NICE guidelines take the spotlight in first aortic session
Anthony Strzalek2020-05-18T18:09:13+01:00World-leading vascular surgeon, Stéphan Haulon (Paris, France), who is also a member of the CX Aortic Executive Board, extols the “high-quality” digital educational programme of CX 2020 LIVE and highlights the “very exciting” thoracic aortic and abdominal aortic consensus sessions that will be chaired by a host of other experts […]
COVID-19: No evidence of increased aortic emergencies in Lombardy
Anthony Strzalek2020-05-19T10:33:36+01:00Roberto Chiesa (Milan, Italy), who was at the frontline of the COVID-19 pandemic in the Lombardy region of Italy, shares his team’s experience in tackling vascular and aortic emergencies during this time.
Chiesa outlines the response in the region to the pandemic, including the creation of new intensive care units (ICUs) […]
Paclitaxel controversy to headline inaugural CX 2020 LIVE session
Anthony Strzalek2020-07-09T23:32:08+01:00
Join a wealth of experts and faculty from around the world for the very first session of CX 2020 LIVE—the inaugural virtual Charing Cross (CX) Symposium—titled ‘Paclitaxel-coated Device Consensus Update’.
Renowned interventional radiologist Andrew Holden (Auckland, New Zealand), who is a member of the CX Peripheral Arterial Executive Board, welcomes the […]
CX 2020 LIVE: Charing Cross Symposium unveils live, interactive digital event
Anthony Strzalek2020-05-13T17:13:26+01:00The Charing Cross (CX) Symposium, with its world-class faculty and unique focus on live audience participation, has announced the launch of a not-to-be missed and timely vascular and endovascular education virtual event: CX 2020 LIVE. Mark your calendars for this live digital experience, designed to deliver high-quality vascular education and featuring […]
CX 2020 is cancelled due to COVID-19
cxsymposium2020-04-21T15:03:24+01:00Dear colleagues,
The CX Symposium team has made the difficult but necessary decision to cancel the Charing Cross Symposium 2020 that was scheduled to take place from 21 to 24 April in London, UK. We will be refunding in full all the registration fees incurred by delegates and will work with our Faculty members to assist […]
Number of vascular surgeons being trained is “woefully inadequate”
Anthony Strzalek2020-03-09T14:43:42+00:00Sophie Renton (London, UK) moderates a session with Michel Makaroun (Pittsburgh, USA) and Armando Mansilha (Porto, Portugal) where the trio discuss some of the challenges relating to recruitment within the field of vascular surgery.
Makaroun explains that there are two main recruitment pathways into vascular surgery in the USA but notes […]
CX 2020: What to expect from this year’s Aortic Workshop
Anthony Strzalek2020-03-09T10:48:40+00:00Kevin Mani (Uppsala, Sweden) chats to BLearning about the upcoming Aortic Workshop at CX 2020 (Charing Cross Symposium; 21–24 April, London, UK), noting how the sessions will give attendees access to experts, the latest technologies and provide a platform for hands-on experience with new devices and imaging technology.
Workshop participants can take this […]
Michel Makaroun discusses why the CX Symposium is a “special meeting”
Anthony Strzalek2020-03-09T09:09:07+00:00Roger Greenhalgh (London, UK) speaks to former president of the SVS (2018–2019) Michel Makaroun (Pittsburgh, USA) about the CX Symposium which Makaroun says “always has a special flavour” and is “a special meeting”. Makaroun also touches on some of the similarities and differences between CX and the SVS meeting in […]
Long-term partnership helps drive CX Symposium forward
Anthony Strzalek2020-03-06T09:50:48+00:00Roger Greenhalgh (London, UK) and W L Gore’s Michael Koenke (Flagstaff, USA), take a look back at the history of the Charing Cross Symposium (CX), which began in 1978.
Koenke says that CX is a “special meeting for us”, adding that it is an “opportunity for us to connect with our […]
Scientific level of CX Symposium “very attractive” for attendees
Anthony Strzalek2020-03-06T09:55:51+00:00Roger Greenhalgh (London, UK) chats with Enrico Ascher (New York, USA) about some of the highlights of the CX Symposium, how it differs to the VEITH symposium in the USA and also how the two complement each other.
Ascher says he is always “totally amazed by the scientific level” of CX, […]
CX 2019: A Latin American perspective in vascular and endovascular surgery
Anthony Strzalek2020-03-02T14:30:44+00:00Alberto Muñoz (Bogota, Colombia) talks to BLearning at CX 2019 (Charing Cross Symposium; 15–18 April, London, UK) about how the meeting provides a “great opportunity” to update attendees on the latest in vascular and endovascular surgery.
Muñoz goes on to highlight the international flavour of Charing Cross and notes the importance of the […]
CX 2019: Experts discuss latest findings from the STEP study
Anthony Strzalek2019-11-01T10:14:03+00:00Roger Greenhalgh (London, UK) is joined by Fiona Rohlffs (Hamburg, Germany), Tilo Kölbel (Hamburg, Germany) and Heinz Jakob (Essen, Germany) to discuss the latest findings of the Stroke from Thoracic Endovascular Procedures (STEP) study which were presented at CX 2019 (Charing Cross Symposium; 15–18 April 2019, London, UK). […]
Type II endoleak with secondary sac expansion remains elusive
Anthony Strzalek2020-01-08T13:00:32+00:00Leading experts including Robert Morgan (London, UK), Richard McWilliams (Liverpool, UK), Stéphan Haulon (Paris, France), Tilo Kölbel (Hamburg, Germany) and Peter Schneider (Honolulu, USA) discuss the various approaches to correcting endoleak during CX 2018 (Charing Cross Symposium; 24–27 April 2018; London, UK).
CX 2019: VBX set to be a “game-changer” for treating complex aortic aneurysms
Anthony Strzalek2019-08-20T14:42:29+01:00Giovanni Torsello (Münster, Germany) and Mauro Gargiulo (Bologna, Italy) discuss the “very high” morbidity and mortality caused by open surgery when treating complex aortic aneurysms. This is […]
MDR will be “the most devastating thing to happen to European healthcare since WW2”
Anthony Strzalek2019-09-16T10:41:45+01:00Speaking at the Charing Cross Symposium 2019, Jeffrey Jump (Nyon, Switzerland), outlines the impact of the Medical Device Regulations (MDR), which he believes will be “the most devastating thing to happen to European healthcare in Europe since World War Two”.
Patients “will bear the brunt of this hardship” says Jump, who […]
Intra-arterial calcification poses “one of the biggest challenges” for endovascular therapy
Anthony Strzalek2019-09-06T16:03:07+01:00Andrew Holden (Auckland, New Zealand) provides perspective on the first results from the Disrupt PAD III observational registry that were presented at the Charing Cross International Symposium 2019. This large registry is designed to evaluate the use of Intravascular Lithotripsy (IVL; Shockwave) in the treatment of calcified arteries beyond the […]
Highlights from the CX 2019 session, Paclitaxel: The last word
2019-09-06T16:04:24+01:00The 2019 Charing Cross Symposium (CX; 15–18 April, London, UK) featured an in-depth highlight session on the paclitaxel controversy. […]
With CoveredSeal, Valiant Navion™ offers physicians the “best of both worlds” for TEVAR procedures
Anthony Strzalek2019-09-06T16:05:44+01:00Paul Hayes (Cambridge, UK), Frank Arko (Charlotte, USA) and Fabio Verzini (Turin, Italy) discuss the Valiant Navion™ (Medtronic) live thoracic endovascular aortic repair (TEVAR) case at Charing Cross 2019, which demonstrated the use of the new CoveredSeal proximal configuration.
Hayes talks about the accuracy of the device’s deployment around the thoracic […]
With focal dissection repair, the Tack Endovascular System “returns control to the operator”
Anthony Strzalek2019-09-06T16:08:00+01:00Andrew Holden (Auckland, New Zealand) and Peter Schneider (San Francisco, USA) discuss the Tack Endovascular System (Intact Vascular) and its impact on below the knee and above the knee procedures at the Charing Cross Symposium 2019. Schneider talks about the “limitations of the current clunky stenting paradigm” and how the […]
Durability of endovascular aneurysm repair and the NICE guidelines
Anthony Strzalek2019-09-06T16:08:53+01:00A CX 2019 special session highlighted the durability of endovascular aneurysm repair (EVAR) and the implications of the UK National Institute for Health and Care Excellence (NICE) draft aortic aneurysm guidelines. The final publication of these guidelines has been postponed on numerous occasions.
Opening the session, Stéphan Haulon and his team […]
Optimising surveillance and reintervention strategy following elective EVAR
Anthony Strzalek2019-09-06T16:09:48+01:00Roger Greenhalgh (London, UK) interviewed Fiona Rohlffs (Hamburg, Germany), Maarit Venermo (Helsinki, Finland) and David Epstein (Granada, Spain) at the CX Live studio after their ‘Optimising surveillance and reintervention strategy following elective EVAR’ session.
Medical device industry leaders call for three big changes to “devastating” EU MDR legislation
cxsymposium2019-11-21T11:56:06+00:00Industry leaders say approximately 50% of all medical devices will be withdrawn from the market; around 30% of manufacturers will not survive and patients might be at risk as a result of the European Medical Device Regulation (EU MDR), which is described by some as the “most disruptive force […]
New APERTO AVF RCT data show DCB reduces restenosis significantly more than high-pressure balloon alone
Anthony Strzalek2019-09-06T16:10:43+01:00Matteo Tozzi (Varese, Italy) discusses new data from the APERTO AVF study, the first randomised trial in China to compare the safety and efficacy of the APERTO drug-coated balloon (DCB; Cardionovum) with a high-pressure balloon. The trial results for the treatment of failing haemodialysis arteriovenous shunts were presented by Qizhuang […]
Comprehensive innovation programme gives insights into the technologies of the future
dawn2019-09-26T15:00:31+01:00The CX Innovation Programme showcased technological developments happening in all areas of the Charing Cross Symposium: acute stroke, aortic, peripheral, venous, vascular access, and wound care. Additionally, a new “Dragons’ Den” winner was crowned.
In the first talk of the session, Lindsay Machan (Vancouver, Canada) talked about the lessons he had learnt from being a founder […]
TEVAR performed outside the IFU has “dramatically worse outcomes”
Anthony Strzalek2019-09-06T16:11:31+01:00Dittmar Boeckler (Heidelberg, Germany) tells CX Live that treating patients with thoracic endovascular aortic repair (TEVAR) outside the instructions for use (IFU) has a dramatic effect on patient outcomes after the procedure. “We observed a significant increase in mortality […] an increased reintervention rate and four times higher endoleak rate,” […]
New MIMICS-2 trial data show helical stents provide durable treatment of diseased femoropopliteal arteries
cxsymposium2019-09-06T16:12:16+01:00 New data from the MIMICS-2 trial show the BioMimics 3D stent, which mimics natural vascular curvature, remains safe and effective for patients with symptomatic atherosclerotic disease of the femoropopliteal arteries two years after implantation.
New data from the MIMICS-2 trial show the BioMimics 3D stent, which mimics natural vascular curvature, remains safe and effective for patients with symptomatic atherosclerotic disease of the femoropopliteal arteries two years after implantation.
Principal investigator Timothy Sullivan (Minnesota, USA) presented the two-year findings at this year’s Charing […]
Multicentre dataset reveals the added value of diffusion-weighted imaging in TEVAR practice
cxsymposium2019-09-06T16:13:28+01:00
Novel data from the Stroke from Thoracic Endovascular Repair (STEP) collaborators has provided insight into current practice of thoracic endovascular aortic repair (TEVAR), and the future role of diffusion-weighted magnetic resonance imaging (DW-MRI) in reducing stroke from endovascular repair. The multicentre dataset was presented at a Charing […]
Endovascular repair matches open surgery for treatment of men with complex but low-risk aortoiliac disease using self-expanding covered stents
cxsymposium2019-04-18T17:16:33+01:00 Michele Antonello
Michele Antonello
Endovascular repair shortens hospital stay and has comparable outcomes to open surgery when balloon-expandable covered stents are used to treat low-risk male patients with complex aortoiliac lesions, new data show. Michele Antonello (Padua, Italy), described some of the study’s key results during a presentation at […]
0.018-inch drug delivery platforms are as effective as 0.035-inch devices
cxsymposium2019-09-06T16:14:14+01:00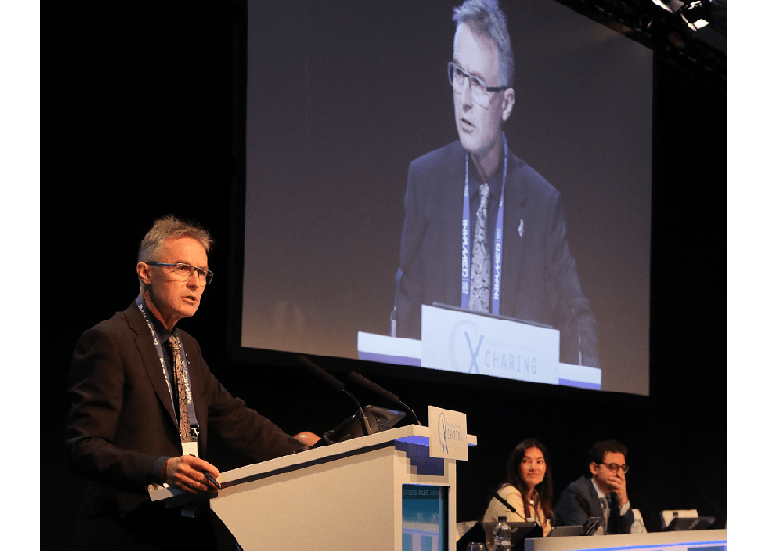 Andrew Holden
Andrew Holden
Interventionists can confidently use lower profile drug delivery platforms, based on data that shows 0.018-inch guidewire devices are non-inferior to 0.035-inch guidewires. In a Podium 1st presentation at Charing Cross (CX) Symposium on Thursday, Andrew Holden (Auckland, New Zealand) said: “At 90 days, we can […]
Live case of challenging thoracic endovascular repair demonstrated using virtual reality at CX
cxsymposium2019-11-25T14:50:39+00:00
Tilo Kölbel’s team, led on site in Hamburg, Germany, by Giuseppe Panuccio, successfully performed a thoracic endovascular aortic repair (TEVAR) on a 77-year old female patient with American Stroke Association (ASA) class III, and multiple aortic aneurysm disease with multiple aneurysms. The live case was viewed by […]
CX 2019: Highlights from the inaugural iWounds Workshop
Anthony Strzalek2019-09-06T16:16:14+01:00Michael Edmonds (London, UK), Thomas Serena (Warren, USA), Keith Harding (Cardiff, UK), and Williams Ennis (Oak Lawn, USA) talk at CX Live about the first ever iWounds workshop, the importance of wound management and what they hope to achieve.
Edmonds discusses why “too many legs are being lost” and Serena talks […]
Endoanchors add to therapy options for AAA
cxsymposium2019-04-18T10:09:07+01:00 Frank Arko
Frank Arko
Using EndoAnchors to fix and seal endovascular aortic grafts is safe and effective for short-neck abdominal aortic aneurysm (AAA) patients, two-year data from the ANCHOR global registry have shown. Frank Arko (Charlotte, USA) presented the findings yesterday at CX Symposium in a podium first session, […]
The impact of paclitaxel concerns on vascular access
cxsymposium2019-04-18T10:32:43+01:00
As part of Wednesday’s CX Vascular Access Workshop in the Pillar Hall Learning Centre, there was a section of the programme on drug-coated balloon trial updates which ended with a panel discussion on whether peripheral vascular disease data is applicable to vascular access. The discussion […]
EVAR with an iliac branch endoprothesis is safe and effective
cxsymposium2019-09-06T16:20:32+01:00 Fabio Verzini
Fabio Verzini
Yesterday, during the Aortic Podium 1st session, Fabio Verzini (Turin, Italy) reported that endovascular aneurysm repair (EVAR) with an iliac branch endoprothesis (Gore) is safe and effective. He added that the procedure was associated with improved quality of life in the short term and […]
Virtual reality stimulates discussion at CX
cxsymposium2019-04-18T10:30:36+01:00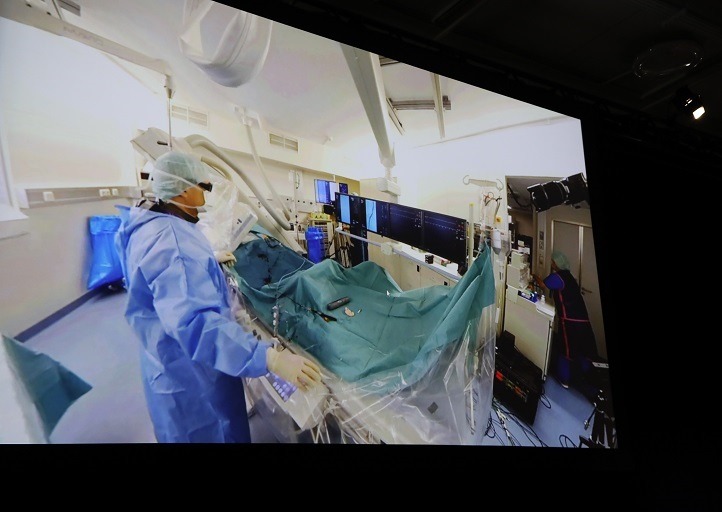
For the first time, the Charing Cross (CX) audience has been able to experience an array of virtual reality live cases in both the peripheral and aortic programmes. The virtual reality live streaming in 360° video, accompanied by a running commentary by the operator, provided a […]
EVAR funding threatened, while strategy for safe, cost-effective repair is “just around the corner”
cxsymposium2019-04-18T09:59:05+01:00
The durability of endovascular aneurysm repair (EVAR) is in the limelight as the UK vascular community awaits the final aortic aneurysm guidelines from the National Institute for Health and Care Excellence (NICE), set to go against widespread endovascular practice in recommending open repair over EVAR. In May […]
Vascular recruitment a “universal problem”: The global perspective
cxsymposium2019-04-18T10:04:27+01:00 Sophie Renton
Sophie Renton
Across the globe, vascular recruitment is struggling. This was the reason why delegates convened in the Upper Auditorium yesterday afternoon for a CX Highlight Session dedicated to the topic, where representatives from three continents explained exactly how and why the vascular specialty is experiencing this […]
Don’t miss: The influence of hyperglycaemia on spinal cord injury
Anthony Strzalek2019-09-06T16:21:31+01:00Jade Hiramoto (San Francisco, USA) tells CX Live about why she believes “everyone should consider normalising glucose levels after long, complex endovascular aortic procedures—and maybe even after open thoracoabdominal aortic procedures.”
Hiramoto is presenting new research on this topic at the Charing Cross session on Spinal Cord Ischaemia in the Upper […]
CX 2019: Why a signed consent form “is not consent”
Anthony Strzalek2019-09-06T16:22:57+01:00Jonathan Beard, (Sheffield, UK), discusses the current medicolegal issues in the field and explains how vascular surgeons can become “the fall-guy for the inadequacies of other clinicians”. Beard touches on how the threshold for informed consent has changed “dramatically” in recent years which has led to “the death of […]
News from CX 2019: Evidence supports safety of paclitaxel-coated devices
Anthony Strzalek2019-09-06T16:23:55+01:00At the Charing Cross International Symposium, Gary Ansel (Columbus, Ohio) moderates a global panel that includes Thomas Albrecht (Berlin, Germany), Peter Schneider (San Francisco, USA) and Eric Secemsky (Boston, USA). The panel outlines the value of paclitaxel devices in the treatment of peripheral arterial disease patients; examines new evidence on […]
Breaking news from CX 2019: Cook leads the way to data transparency with release of long-term ZILVER PTX patient-level data
Anthony Strzalek2019-09-06T16:27:30+01:00There were several calls at the CX 2019 Highlight Session Paclitaxel: The last word on the need for a meta-analysis of individual patient-level data going forward and greater transparency and sharing of the available randomised controlled trial data of paclitaxel-coated devices.
Cook Medical has just announced the release of de-identifiable […]
Key take-aways from the CX 2019 Highlight Session on Paclitaxel: The Last Word
Anthony Strzalek2019-09-06T16:28:17+01:00Elena Ladich (Hollywood, USA) and Roger Greenhalgh (London, UK) discuss the current uncertainty surrounding the use of paclitaxel devices and the need for individual patient-level data analyses going forward to better understand the mortality signal raised by the Katsanos et al meta-analysis.
Ladich believes that “we need to take a step […]
While CX audience deems paclitaxel not dangerous, vascular pathologist says establishing “truth takes time”
cxsymposium2019-09-06T16:29:29+01:00 Elena Ladich
Elena Ladich
Delegates voted overwhelmingly against the notion that there was a demonstrable danger in any organ of the body attributed to circulating paclitaxel. These polling results, with a 67% majority, came after vascular pathologist Elena Ladich (Hollywood, USA) presented insights from new analysis on the effects […]
CX highlights Acute Stroke Challenges
cxsymposium2019-09-06T16:30:22+01:00 On Monday, the 2019 Charing Cross (CX) Symposium opened with the Acute Stroke Challenges programme; the session, which was chaired by Barbara Rantner (Innsbruck, Austria), Hugh Markus (Cambridge, UK) and Ross Naylor (Leicester, UK), featured five CX debates, Hot carotid Challenges, intracranial thrombectomy, and stroke after […]
On Monday, the 2019 Charing Cross (CX) Symposium opened with the Acute Stroke Challenges programme; the session, which was chaired by Barbara Rantner (Innsbruck, Austria), Hugh Markus (Cambridge, UK) and Ross Naylor (Leicester, UK), featured five CX debates, Hot carotid Challenges, intracranial thrombectomy, and stroke after […]
Ambulatory treatment can be offered to high-risk patients too
cxsymposium2019-09-06T16:31:54+01:00Office-based lower extremity arterial interventions are feasible, safe, and cost-effective, and provide high levels of patient satisfaction. Enrico Ascher (Brooklyn, USA) presented data that “challenges the dogma that high-risk patients should not be offered office-based endovascular infrainguinal arterial procedures”. He was providing a US perspective on ambulatory interventions at CX Symposium 2019.
Ascher cited studies that […]
“Urgent action is required for patients with venous ulceration”
cxsymposium2019-04-16T21:56:16+01:00 Alun Davies
Alun Davies
Despite what current guidelines denote in addition to the emergence of data supporting the early intervention of venous ulceration, Alun Davies (London, UK) put forward that the recommendations are consistently being ignored, and highlighted that: “Urgent action is required to improve referral pathways between primary […]
Appropriate follow-up of patients after endovascular procedures
cxsymposium2019-04-16T21:30:59+01:00Marianne Brodmann outlines follow-up and surveillance protocols after endovascular treatments for peripheral arterial disease (PAD), arguing for a standardised approach to ensure best practice for patients who have gone through peripheral endovascular procedures.
Endovascular procedures have become a key element of treating patients with PAD, either with intermittent claudication or critical limb […]
VenaSeal maintains safety and efficacy at extended five-year follow-up
dawn2019-04-16T21:52:45+01:00 Nick Morrison
Nick Morrison
The first-ever 60-month data on Medtronic’s VenaSeal closure system were presented on Tuesday, indicating that at five years, treatment with the cyanoacrylate adhesive for closure of diseased vein segments was not inferior to the alternative treatment arm of radiofrequency ablation (RFA). Nick Morrison of […]
CX 2019: Stroke following cardiac surgery is a “wake-up call”; safety of carotid stenting debate
Anthony Strzalek2019-09-06T16:33:28+01:00In the Acute Stroke Challenges session on Monday, Nick Hopkins talks to CX Live about the “significant increase” of incidents of stroke following cardiac surgery which is “much more common than previously thought”.
Ross Naylor and Peter Schneider debated the impact of new technologies and innovations making carotid artery stenting as […]
CX 2019: Programme Guide
cxsymposium2019-09-06T16:34:23+01:00Read the programme guide below or visit the CX 2019 online programme for the most up-to-date timings.
Barry Katzen looks forward to CX 2019
cxsymposium2019-09-06T16:35:37+01:00Register now to join him and the global vascular community at CX 2019!
What is new @ CX 2019?
cxsymposium2019-09-06T16:40:25+01:00
The last word on the paclitaxel controversy
The meta-analysis of Katsanos et al in JAHA suggests the need for a reappraisal of paclitaxel-coated balloons and paclitaxel-eluting stents. CX 2019 will carry out an in-depth independent review of the benefits of paclitaxel in reducing restenosis versus the […]
CX 2019: NICE AAA guidelines under the spotlight
cxsymposium2019-09-06T16:42:13+01:00
The publication of the draft aortic guidelines by the National Institute for Health and Care Excellence (NICE) in May 2018 raised quite a stir.
However, the story is not over. In a CX Special Session, new analysis will show what we now know about long-term EVAR outcomes, and the vascular community’s concerns over the […]
CX 2019: The last word on the paclitaxel controversy
2019-02-07T17:15:34+00:00Since the publication of Katsanos et al’s meta-analysis in the Journal of the American Heart Association (JAHA), the science and support for paclitaxel-releasing devices in the legs is an unfolding story.
A CX 2019 Highlight session will feature two hours of independent review of the data and in-depth debate and discussion.
CX […]
CX Symposium provides “ideal opportunity” to share your research
cxsymposium2019-09-06T16:45:12+01:00In this interview, Meryl Davis, chair of the CX Abstract Board, talks about the benefits of presenting research at the CX Symposium and the importance of CX as a “global village”.
The CX Abstract Board is calling for senior and trainee doctors in the vascular and endovascular field to submit their […]
CX 2020 Abstracts
dawn2019-11-22T13:30:34+00:00
Abstract Submissions for CX 2020 are now closed
The CX Abstract Board is calling for senior and trainee doctors in the vascular and endovascular field to submit their abstracts for presentation at the Charing Cross Symposium (21–24 April 2020, London, UK).
The best abstract presentation […]
iWounds at CX Symposium
cxsymposium2019-09-06T16:47:44+01:00Download the iWounds at CX Symposium brochure to get all the details:
https://www.cxsymposium.com/wp-content/uploads/2018/07/iwounds-a4-4pageflyer-LR.pdf
CX 2018: Podium 1st presentations
cxsymposium2019-09-06T16:48:54+01:00In addition to the plethora of late-breaking trials that were unveiled at this year’s Symposium, there was a vast array of Podium 1st presentations. These presentations showcased novel techniques and technologies, provided further insights into the safety and effectiveness of established devices, and highlighted potential solutions to current challenges in the management of vascular disease.
Peripheral […]
CX Abstract and Poster Prize winners announced!
dawn2019-09-30T11:51:58+01:00 Poster prize winner Wei-Wen Ang with CX Abstract Board and Poster member Anna Prent
Poster prize winner Wei-Wen Ang with CX Abstract Board and Poster member Anna Prent
At Charing Cross 2018, abstracts were presented—across 17 sessions—on the five programme areas of CX: Acute stroke, Thoracic Aortic Abdominal Aortic, Peripheral, Venous, and Vascular Access. Additionally, throughout the symposium, delegates […]
CX 2018 in numbers
cxsymposium2019-09-09T09:13:00+01:00
CX 2018 in numbers
This year’s CX welcomed more than 4,000 participants at Olympia London from a record-breaking 86 countries.
A leader of innovation, CX 2018 hosted a record-breaking 71 exhibitors and 15 new product launches—similar numbers are expected for CX 2019.
See the full stats and highlights in the video above.
CX showcases most promising vascular innovations
cxsymposium2019-10-29T10:22:37+00:00 CX 2018 Dragons’ Den winner, Kieran Murphy, with the Dragons
CX 2018 Dragons’ Den winner, Kieran Murphy, with the Dragons
The Innovation Showcase yesterday brought together the CX values of education, innovation, and evidence to paint a global picture of vascular entrepreneurship, highlighting the controversies currently threatening the next generation of game-changing products.
Beginning the […]
One-year outcomes of EndoAnchor study adds ESAR—endosuture aneurysm repair—to the endovascular lexicon
dawn2018-04-27T10:01:22+01:00 Frank Arko
Frank Arko
Frank Arko (Charlotte, USA) presented one-year results from the 70-patient short-neck cohort of the ANCHOR Registry. Patients were treated with an endograft and endoanchors (Endurant and Heli-FX, Medtronic) for abdominal aortic aneurysm. The data showed “very good clinical outcomes in a challenging patient population”, Arko […]
Low early mortality and high technical success for novel thoracic stent graft
dawn2018-04-27T10:33:59+01:00
A physician-sponsored investigational device exemption trial on thoracic endovascular aortic repair (TEVAR) using a new thoracic stent graft (Valiant thoracoabdominal aortic aneurysm stent graft, Medtronic), along with its initial 30-day data, was revealed at a Podium 1st presentation at CX 2018 by Thomas Maldonado (New York, USA). […]
First case experience presented with next generation conformable EVAR device
dawn2018-04-27T09:29:05+01:00
CX delegates were presented data from the first 10 EVAR patients treated using a new conformable device, the Gore Excluder conformable abdominal aortic aneurysm endoprosthesis with active control system, yesterday.
Robert Rhee (Brooklyn, New York), the national principal investigator of the US investigational device exemption (IDE) trial for […]
“Fantastic” live case demonstrates potential value of intravascular lithotripsy
dawn2018-04-27T09:56:24+01:00
In an exciting session yesterday, the CX audience witnessed the demonstration of a novel treatment strategy with intravascular lithotripsy for calcified arterial stenosis. It will now become a CX tradition to feature live cases to showcase what has been discussed in the presentations, so that the audience […]
Live cases to illustrate the data: The future of the Charing Cross Symposium
dawn2018-04-27T09:06:25+01:00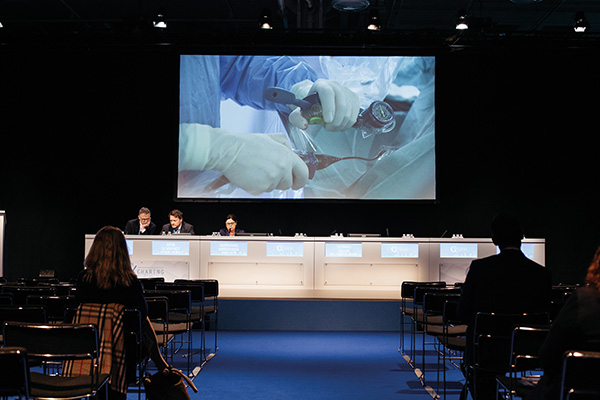
In yesterday’s plenary programmes, the future of the Charing Symposium was launched with the initiation of live case demonstrations to illustrate key data discussed on the programme. This year, both the Thoracic Aortic programme and the Peripheral Critical Ischaemia programme featured these illuminating cases, which stirred discussion and […]
STEP seeks to advance patient safety after TEVAR
dawn2018-04-27T10:06:45+01:00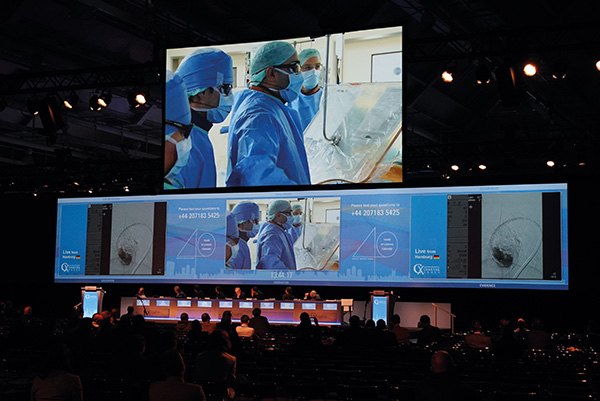
Stroke is a major concern following TEVAR (thoracic endovascular aortic repair) and Charing Cross delegates heard the results from a collaborative study that pooled practice data from a number of high-volume centres in order to benefit patients undergoing the procedure. A highlight of the session was the […]
“Promising” initial results for sirolimus-eluting bioresorbable scaffold in DESappear trial
cxsymposium2018-04-25T11:15:59+01:00 Andrew Holden
Andrew Holden
The sirolimus-eluting bioresorbable peripheral scaffold system (Prava; Elixir Medical) is designed to treat stenoses and occlusion in the superficial femoral artery. Prava was first implanted in October 2016 and will be clinically evaluated in the DESappear trial that has currently enrolled 21 patients at 11 […]
CX 2018: Landmark EVRA trial provides first Level 1 evidence for early endovenous ablation
dawn2018-04-25T10:25:09+01:00
The first full results of the Early Venous Reflux Ablation (EVRA) ulcer study were presented at the Charing Cross Symposium yesterday. The presentation was accompanied by simultaneous publication in the New England Journal of Medicine after the session.
The randomised trial revealed that early endovenous ablation outperforms deferred […]
Majority of CX audience decides endovascular-first strategy for critical limb ischaemia is a concept without evidence
dawn2018-04-25T10:26:13+01:00
At the CX Great Debate held yesterday, vascular and endovascular surgery titans went head-to-head to debate the contentious topic “Endovascular-first strategy for critical limb ischaemia is a concept without evidence”. Arguing in favour of this motion were current Society for Vascular Surgery (SVS) president Clement […]
Drug-coated balloon is “highly effective and safe” and shows outstanding clinical improvement for patients compared to an uncoated balloon
dawn2018-04-25T10:49:59+01:00 Ulf Teichgraeber
Ulf Teichgraeber
The 12-month results from the full clinical cohort of the EffPAC randomised controlled trial, were presented for the first time at the CX Symposium yesterday.
Target lesion revascularisation at 12 months was 1.3% (vs 17.7% in the plain angioplasty group, p<0.001). Primary patency at 12 months […]
Paclitaxel-eluting stent fails BATTLE against bare metal stent
dawn2018-04-25T10:29:09+01:00 Yann Gouëffic
Yann Gouëffic
The BATTLE trial comparing a drug-eluting stent (Zilver PTX, Cook) vs. a bare metal stent (Misago, Terumo) for the treatment of intermediate femoropopliteal lesions has failed to the show superiority of the paclitaxel-coated stent at one-year follow-up. The trial highlights a need for further direct […]
EVRA (Early Venous Reflux Ablation) Ulcer Trial presented for the first time at the Charing Cross Symposium
cxsymposium2019-09-09T09:23:21+01:00Alun Davies, EVRA chief investigator
Imperial College, London, United Kingdom
CX 2018: Record number of hands-on workshop opportunities for those technical tips and tricks!
dawn2019-09-09T09:24:57+01:00This year, there are four CX Workshops that offer a record number of hands-on workshop opportunities for those technical tips and tricks. The CX Venous Workshop—which has now been running for 10 years—has more than 80 workstations over two days. Last year, a staggering 1,100 people attended this workshop. Located in the Venous City, […]
CX@CEC: Focus on challenging aortic disease
dawn2019-09-09T09:26:03+01:00 Rodney White
Rodney White
This year’s annual China Endovascular Course (CEC; 2–5 November, Beijing, China) played host to the first CX@CEC session, in collaboration with the Charing Cross Symposium. Focused on complex aortic disease, this session featured a selection of international and Chinese experts […]
CX 2018: Acute Stroke to explore the greatest carotid controversies of the day
dawn2018-03-27T17:26:38+01:00 Ross Naylor at CX 2017
Ross Naylor at CX 2017
CX 2018 will close with the Acute Stroke CONTROVERSIES programme, which Ross Naylor—a member of the CX Programme Organising Board—promises will be a memorable session. The highlight will be five debates that will address a few “sacred cows” that arouse considerable controversy around […]
CX 2018: 40 Years of Looking Forward with vascular education as it should be!
dawn2019-09-09T09:27:16+01:00This year, CX celebrates 40 Years of Looking Forward. In this video, members of the CX Programme Organising Board and the CX Faculty explain why CX is not only unique but also provides vascular education “as it should be”! Register now for CX 2018!
[…]
CX 2018: “I believe delegates of CX 2018 should attend this presentation since the information provided by it will change their practice”
dawn2019-09-09T09:29:42+01:00
Juan Parodi (San Isidro, Argentina) gives an opening talk at CX 2018 “Reversal of lower extremity intermittent claudication and rest pain by hydration”. Come to CX on Day 1 to see if it as simple as that! Read the interview below for more information.
What are the limitations […]
The opportunity for discussion is a key benefit of CX
dawn2018-02-15T14:35:40+00:00Fabrizio Fanelli (Florence, Italy), CX Faculty, says that a key point about CX is that it – unlike other meetings – provides opportunities for discussion. He notes that the “active discussion” at the podium is “very important” for what you learn at the meeting. Register for CX 2018!
[…]
CX has had “a very significant impact on my practice”
dawn2018-04-05T17:53:26+01:00CX Faculty member Barry Katzen (Miami, United States) discusses how attending CX has affected his clinical practice and how he always is “universally losing” debates at CX! Register for CX 2018!
“Excellent” is the word Prof Davies uses to describe CX
dawn2018-02-15T14:27:22+00:00CX programme organising board member Alun Davies (Imperial College, London, United Kingdom) reviews how CX has changed in the last 24 years that he has been attending the symposium, his most memorable moments, and the one word he would use to describe the meeting. Register for CX 2018!
[…]
“Every time I come to CX, I learn more and more”
dawn2018-02-15T14:20:26+00:00“One of the great things” about CX, according to CX Faculty member Kathleen Gibson (Bellevue, United States), is that it provides a “global perspective”. She says she “learns more and more” every time she attends. Register for CX 2018!
CX is “a very good forum” for new technologies
dawn2018-02-15T13:13:21+00:00Rodney White (Torrance, United States), CX Faculty, says that CX has been a “very good forum” for not only new technologies but also how we adapt them to current practice. He notes that CX chairman Roger Greenhalgh has always been at the forefront of the latest developments. Register for CX 2018!
The CX Venous Workshop is the best in the world
cxsymposium2020-01-30T16:27:02+00:00Lowell Kabnick (New York, United States), who is a CX Faculty member, describes how the CX Venous Workshop has gone from being a small intimate session to one that, while still intimate, is “really large and all inclusive”. He adds that he believes that it is the best he has seen.
[…]
Meryl Davis discusses the benefits of presenting at the CX Abstract sessions
cxsymposium2019-09-09T09:32:36+01:00Meryl Davis, member of the CX Abstracts Board, talks about the invaluable opportunity for senior and trainee vascular and endovascular clinicians to present at the CX Abstract sessions.
CX Abstract and Poster Presentations 2017
cxsymposium2019-09-09T09:33:58+01:00Over four days, nine abstract and poster sessions covered topics including thoracic aortic, abdominal aortic, peripheral arterial, acute stroke and vascular access. Over 220 abstracts were presented by trainee and senior clinicians from 34 different countries.
 Fadi Taher
Fadi Taher
Fadi Taher (Vienna, Austria) won the best Trainee Clinician Abstract […]
Comprehensive approach to saving limbs outlined at CX
cxsymposium2019-09-09T09:41:12+01:00
Yesterday, the CX ilegx Interdisciplinary Consensus on Severe Ischaemia explored all stages of managing severe limb ischaemia—looking at diagnosis, diabetic patients, and treatment options. This is in keeping with the ilegx’s goal of reducing the rate of amputations and tissue loss related to ischaemia.
The first session of […]
CX Venous Workshop provides “essential ingredients” for the proper treatment of patients
cxsymposium2019-09-09T09:43:18+01:00
Day Two of the CX Venous Workshop showcased the latest technological advancements in the deep venous arena. Building on the success of Wednesday’s first-ever CX Venous Edited Cases, yesterday’s workshop included four more standing-room only expert case presentations looking at venous endovascular stenting and thrombectomy, as well […]
“Phenomenal range of technologies” at CX Innovation Showcase
cxsymposium2019-09-09T09:44:12+01:00
In traditional fashion, the CX Innovation Showcase did the job of raising important questions and challenges in the ever-developing and changing vascular and endovascular field. Chairmen Stephen Greenhalgh (London, UK) and Andrew Holden (Auckland, New Zealand) put together a far-reaching programme covering innovation and regulatory challenges, the […]
CX Vascular Access Skills Course among “the best in Europe”
cxsymposium2019-09-09T09:46:09+01:00
Described as “one of the best” courses of its kind in Europe, this year’s CX Vascular Access Skills course took delegates through each stage of the patient journey, from diagnosis, to treatment, to complication response, through a series of 20 specialist training stations.
“Our main aim was […]
CX salutes Edward B Diethrich – an endovascular pioneer
cxsymposium2019-09-09T09:47:45+01:00
There was a special session convened yesterday at CX to appreciate and recognise Edward B Diethrich’s life, untrammelled spirit, masterful surgical competence, and enormous influence in the cardiovascular field. The internationally esteemed cardiovascular surgeon, inventor, and philanthropist died on 23 February 2017 at the age of 81. […]
Call for an advance in the treatment of aortic dissections at CX
cxsymposium2019-09-09T09:48:42+01:00
Experts in the Thoracic Aortic Plenary sessions yesterday discussed cutting-edge topics that lack support from strong data, making consensus difficult to achieve. Many unanswered questions were raised such as which patients with thoracic disease should get early vs. late treatment; whether centralisation of aortic services would tackle […]
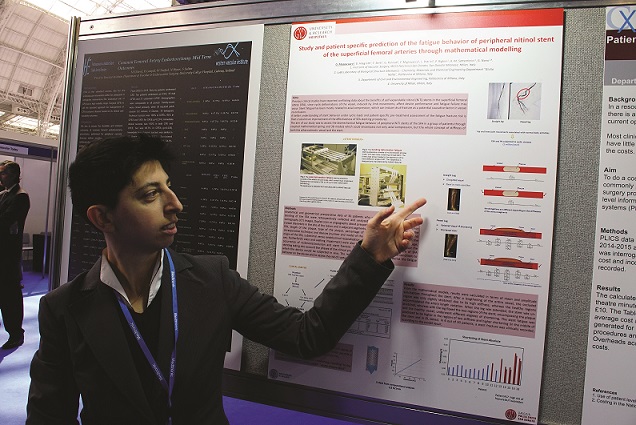
Stent fatigue mathematical modelling research wins CX Selected Posters Session
cxsymposium2019-09-09T09:49:53+01:00
Twelve posters selected for presentation out of 68 posters displayed were carefully reviewed by the CX Abstract Board, who decided to award their prize of a free CX 2018 registration to Daniela Mazzaccaro (San Donato Milanese, Italy) for her poster, “Study and patient specific prediction of the […]
“Fantastic” new Venous City offers “dynamic, informative” hands-on education
cxsymposium2019-09-09T09:50:46+01:00
The CX Venous Workshop returned on Wednesday, bigger than ever before. With a new location in the Exhibition Hall and its own series of edited live case presentations, it continues to showcase innovative venous technology and offer delegates the chance to get hands-on with the latest advances […]
Spotlight on non-thermal varicose vein treatments in first-ever CX Venous Edited Cases
cxsymposium2019-09-09T09:51:50+01:00
For the first time, Day One of this year’s CX Venous Workshop included two edited case presentations (CX Venous Edited Cases). The standing-room only session gave delegates the opportunity to watch two innovative devices in action and quiz experienced surgeons on their top procedural tips.
Chaired by […]
Screening women for abdominal aortic aneurysm would yield little benefit and not be cost-effective
cxsymposium2019-09-09T09:55:30+01:00
First-time data presentation from the SWAN (Screening Women for Abdominal aNeurysms) project was heard at CX yesterday. The study, which used simulation to evaluate whether inviting women to be screened for abdominal aortic aneurysm would have clinical benefit, or be cost-effective, revealed that such a programme would […]
EVAR 2 very long-term results prompt a call for shared decision-making in frail patients
cxsymposium2019-09-09T09:59:22+01:00
A “podium first” presentation of very long-term follow-up data from the EVAR 2 trials suggests that endovascular aneurysm repair (EVAR) fails to improve all-cause mortality, but reduces aneurysm-related mortality compared to no treatment in abdominal aortic aneurysm patients who are physically ineligible for open repair. The data […]
Münster to London: Live and direct
cxsymposium2019-09-09T10:01:27+01:00
For the third time in CX Symposium history the London-based audience was transported via live video feed to Germany for real-time case presentations. This year CX connected with Arne Schwindt and Theodosios Bisdas in Münster, Germany, for four live peripheral cases alternated with edited cases.
The first live […]
Approaching vascular malformations
cxsymposium2019-09-09T10:02:44+01:00 Andreas Saleh
Andreas Saleh
In Tuesday’s CX Congenital Vascular Malformations session, delegates heard about how approaching congenital vascular malformations with systematic pathways of care and classification systems can greatly assist physicians in achieving consistently successful positive outcomes.
Joe Brookes (London, UK) presented on classification of arteriovenous malformations, charting its “emerging […]
Remembering that “babies are not little adults” at CX Paediatric Vascular Emergencies and Case Presentations
cxsymposium2019-09-09T10:03:33+01:00
The particular complexities of dealing with paediatric vessels were discussed in depth at Tuesday’s varied and informative CX Paediatric Vascular Emergencies and Case Presentations session. Covering topics as diverse as acute ischaemia from pre-term to infant and treatment for battlefield trauma in children, the session was one […]
Watch and learn: CX Aortic Edited Cases
cxsymposium2019-09-09T10:04:36+01:00
Tuesday’s CX Aortic Edited Cases session featured exciting and engaging standing-room only case presentations, including Andrew Holden’s (Auckland, New Zealand) first-in-man implantation of the Ovation Alto (Endologix). Covering thoracic, juxtarenal and abdominal cases, the packed-out session explored innovative techniques, novel devices and a veritable array of practical […]
ATTRACT trial results presented at CX
cxsymposium2019-09-09T10:07:11+01:00 Suresh Vedantham
Suresh Vedantham
Delegates heard yesterday the results of the ATTRACT randomised controlled trial, which showed that the addition of catheter-based intervention to anticoagulation failed to significantly decrease the occurrence of post-thrombotic syndrome in deep vein thrombosis patients who received this treatment strategy when compared to its occurrence […]
CX audience homes in on grey area between drug-coated balloons and stents
cxsymposium2019-09-09T10:09:48+01:00
Yesterday, delegates heard brand new data for drug-coated balloons (DCBs) in “wider use” scenarios such as challenging lesions; one-year results for a novel DCB; and late-breaking data on downstream coating effects of various DCBs. Still, 84% of the CX audience said “no” in response to the question: […]
CX Venous Consensus Update: Take-home messages
cxsymposium2019-09-09T10:10:49+01:00The CX Venous Consensus Update—Plenary Programme took place on the first day of CX 2017 (Tuesday, 25 April). Stephen Black, member of the CX Programme Organising Board, summarises the take-home messages of this session.
CX 2017: Peripheral Arterial Consensus highlights
cxsymposium2019-09-09T10:12:09+01:00The CX 2017 Peripheral Arterial Consensus Update Programme will focus on PATHWAYS OF CARE – whether to intervene, when and at what threshold, method and follow-up.
“Over three days (25–27 April) there will be a comprehensive coverage of the most important aspects of peripheral arterial intervention as well as some important new trial evidence being presented […]
CX 2017: Acute Stroke Consensus highlights
cxsymposium2019-09-09T10:13:08+01:00Interventions in the aorta and manipulations in the aortic arch have become a “potential source of embolisation to the brain,” notes Roger Greenhalgh, Chairman of the CX Programme Organising Board. The Acute Stroke Consensus Plenary Programme will address the scope of this problem and PATHWAYS OF CARE to treat acute stroke from a multidisciplinary perspective including: […]
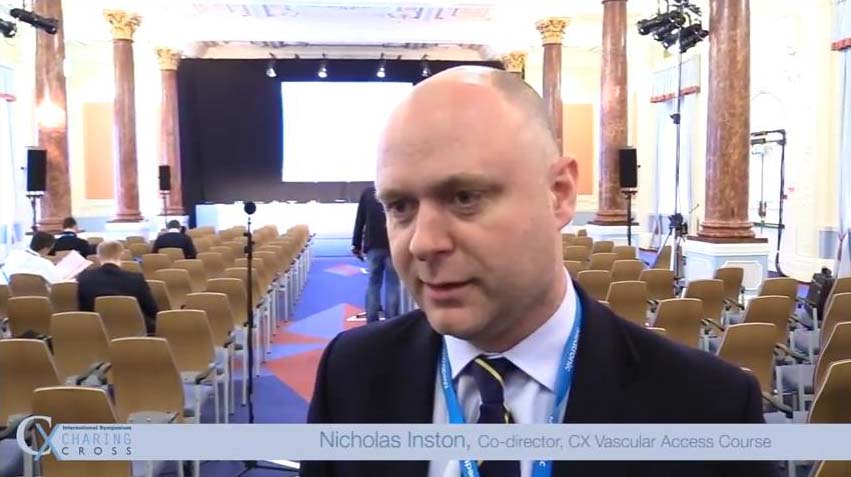
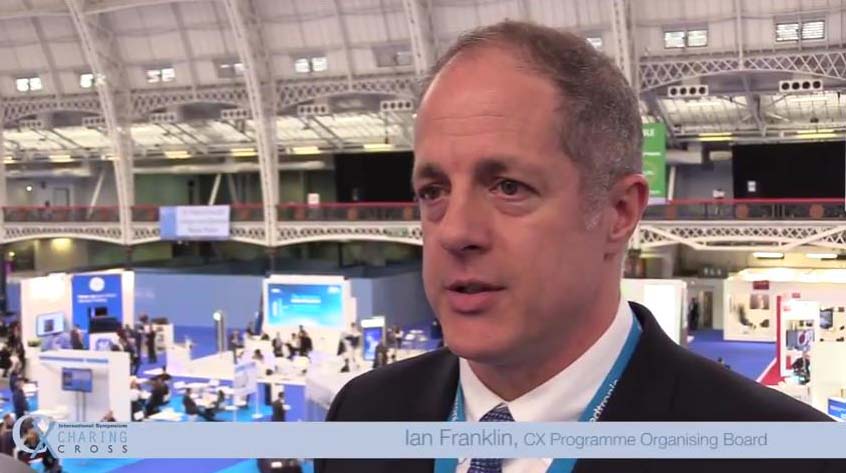
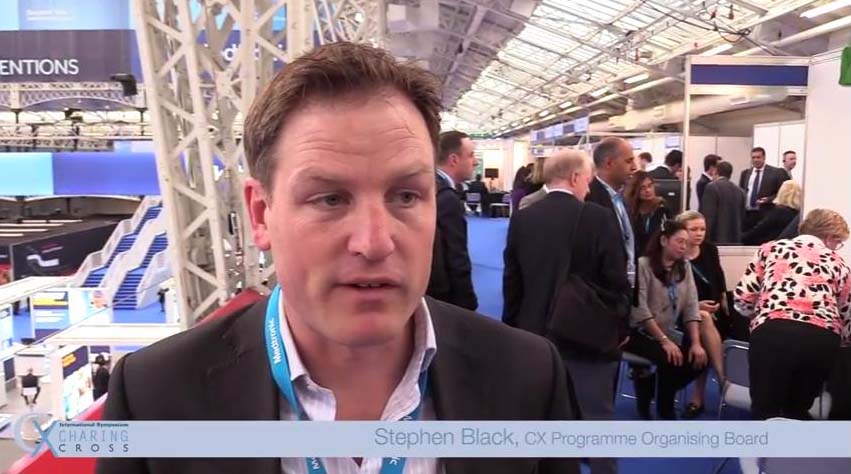
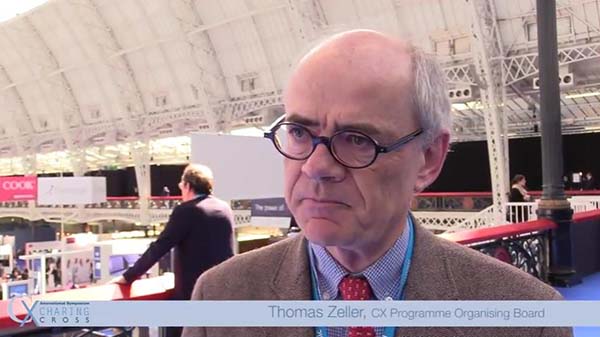
What makes CX so special?
cxsymposium2019-09-09T10:14:37+01:00Vascular and endovascular thought-leaders Andrew Holden, Thomas Zeller, Stephen Black, Ian Franklin, Nicholas Inston, Matt Thompson and Giovanni Torsello share their views on what makes CX so special.
What makes CX so special for you? Tweet your comments @CXSymposium #CXisspecial
Don’t miss CX2017! Click here to […]
CX 2017: Venous Consensus highlights
cxsymposium2019-09-09T10:16:00+01:00The CX 2017 Venous Consensus Update Programme will focus on PATHWAYS OF CARE – whether to intervene, when and at what threshold, method and follow-up, providing delegates with discussions on diagnosis and treatment options for the whole spectrum of venous disease.
Roger Greenhalgh (Chairman, CX Programme Organising Board) discusses with Stephen Black (member, CX Programme Organising […]
CX 2017: Aortic Edited Cases highlights
cxsymposium2019-09-09T10:17:31+01:00Roger Greenhalgh, Chairman of the CX Programme Organising Board, discusses the highlights of the Aortic Edited Cases session at CX 2017.
Complementing the Aortic Plenary Programme, experts in aortic surgery will discuss “tips and tricks” in thoracic, juxtarenal and abdominal aortic reconstructions and also ascending aorta and arch intervention, showing specific techniques and technologies with edited […]
CX 2017: Thoracic Aortic Consensus highlights
cxsymposium2019-09-09T10:20:26+01:00Roger Greenhalgh, Chairman of the CX Programme Organising Board, discusses the highlights of the Thoracic Aortic Consensus Plenary Programme at CX 2017.
Key topics to be discussed include:
- Are the types A and B dissection terms satisfactory?
- Is it time to revisit whether complicated means complicated for type B dissections?
- How does the treatment of complex […]
CX 2017: Abdominal Aortic Consensus highlights
cxsymposium2019-09-09T10:30:30+01:00Roger Greenhalgh, Chairman of the CX Programme Organising Board, discusses the highlights of the Abdominal Aortic Consensus Plenary Programme at CX 2017.
Key topics include:
- Does operating below the threshold of 5.5cms save lives?
- What should you do in the follow-up period after EVAR? How dangerous are type II endoleaks?
- What are the latest results of endovascular […]
CX 2017 “a Plethora of Educational Opportunities”: Matt Thompson on what makes CX so special
cxsymposium2019-09-09T10:33:04+01:00What are the special features of the CX Symposium? Matt Thompson, Co-chairman of the CX Programme Organising Board, highlights “a plethora of educational opportunities” including presentation of new evidence, key messages voiced by opinion leaders, debate and case-based discussion amongst others.
What makes CX so special for you? Tweet your […]
CX 2017 “Brings everyone together”: Nicholas Inston on what makes CX so special
cxsymposium2017-02-14T16:36:26+00:00What are the special features of the CX Symposium? Co-director, CX Vascular Access Course Nicholas Inston (Consultant Surgeon and Clinical Lead for Renal Surgery and Transplantation at Queen Elizabeth Hospital Birmingham, Birmingham, UK) considers the Charing Cross Symposium “brings everyone together” from a worldwide basis and highlights the growing interest in […]
CX 2017 “the Audience is in Charge”: Ian Franklin on what makes CX so special
cxsymposium2017-02-14T16:37:33+00:00What are the special features of the CX Symposium? CX Programme Board member Ian Franklin (Consultant Vascular Surgeon at London Vascular Clinic, London, UK) notes that at CX “the audience is in charge”. “We acknowledge that participants are experts in their own right” and therefore have priority […]
CX 2017 “Access to Innovation”: Stephen Black on what makes CX so special
cxsymposium2017-02-14T16:39:04+00:00What are the special features of the CX Symposium? CX Programme Board member Stephen Black (Consultant Vascular Surgeon at Guy’s and St Thomas’ Hospital, London, UK) highlights “Access to Innovation” and a broad range of modalities as well as the ability to engage closely with the
CX 2017 “the Discussion Culture”: Thomas Zeller on what makes CX so special
cxsymposium2017-02-06T17:42:20+00:00What are the special features of the CX Symposium? CX Programme Board member Thomas Zeller (Professor of Angiology at Albert-Ludwigs University of Freiburg and Head of Department of Angiology at Universitäts – Herzzentrum Freiburg, Bad Krozingen, Germany) considers “the Discussion Culture” and exchange between the CX Faculty and the audience unique.
CX@LINC 2017 ‒ Life after EVAR 1
cxsymposium2019-09-09T10:34:16+01:00CX Faculty members discussed “Life after EVAR 1” at LINC (24‒27 January 2017, Leipzig, Germany). Frans Moll, Co-chairman CX Programme Organising Board, summarises the take-home messages from the session.
Continue the discussion on “Life after EVAR 1” at CX 2017 ( 25‒28 April, Olympia Grand, London, UK). Register here […]
CX is “one of the most prestigious” places for trainees to present abstracts
cxsymposium2019-09-09T10:41:40+01:00Pasha Normahani (Imperial Vascular Unit, Imperial Academic Health Science Centre, London, UK) has been an attendee at the Charing Cross (CX) Symposium for over seven years. In 2016, he presented for the first time at the CX Abstract sessions and was awarded with a Certificate of Merit for his research in peripheral arterial disease. In […]
Frans Moll awarded Honorary Fellowship of the Royal College of Surgeons of Thailand
cxsymposium2019-09-09T10:43:40+01:00 Moll receives the gown and deed from the Minister of Public Health of Thailand
Moll receives the gown and deed from the Minister of Public Health of Thailand
Frans Moll, professor of Vascular Surgery at the University Medical Center Utrecht, Utrecht, The Netherlands, has received an Honorary Fellowship of the Royal College of Surgeons of Thailand (RCST) during the College’s Annual […]
CX 2016 Abstracts winners
cxsymposium2019-09-09T10:45:24+01:00The CX Programme Organising Board and CX Abstract Board congratulate the senior and trainee clinicians who presented their abstracts or posters at the Charing Cross Symposium 2016. In total, over 200 abstracts and posters from 31 countries were presented. Over four days, nine abstract sessions covered vascular […]
Ian Loftus reviews highlights of the CX 2016 Abstract Presentations
cxsymposium2019-09-09T10:50:36+01:00Ian Loftus, Co-chairman CX Abstract Board, reviews the highlights of this year’s CX Abstract Presentations session. He emphasises the quality of the work submitted and how relevant it is for young and senior clinicians to present their research at the Charing Cross Symposium.
Individual Patient Data meta-analysis and EVAR 1 trial results presented at CX
cxsymposium2019-09-09T10:58:46+01:00
The teams of researchers and principal investigators who collected and analysed the data for the Individual Patient Data meta-analysis and EVAR 1 trial presented the data in the session titled “EVAR follow-up and avoidance of secondary sac rupture and death.”
The meta-analysis of Individual Patient Data from […]
Action-packed CX Innovation Showcase provides glimpse into the future
cxsymposium2019-09-09T10:59:31+01:00
According to chair Stephen Greenhalgh, yesterday’s CX Innovation Showcase provided a “glimpse into the future” regarding emerging technologies that address current unmet therapeutic needs. The showcase, which Greenhalgh described as “action packed”, featured presentations on next-generation devices for thoracic aneurysms, abdominal aneurysms, and for peripheral arterial disease.
During […]
CX Venous Workshop provides “unique and productive” introduction to novel techniques
cxsymposium2019-09-09T11:00:26+01:00
Focusing on superficial venous issues, yesterday’s CX Venous Workshop invited delegates to discover new techniques, with a hands-on approach, and speak to experts across the different fields of phlebological practice. Covering techniques from endothermal ablation to diagnostic venous ultrasound, the session was a “unique opportunity” for […]
CX Vascular Access Course inaugural session aims to produce ischaemic steal syndrome consensus
cxsymposium2019-09-09T11:02:28+01:00
The new CX Vascular Access Course began yesterday with a masterclass on vascular access-induced ischaemic steal syndrome. Exploring the mechanisms and pathophysiology of ischaemia in vascular access, the ultimate target of the course was to start the production of a consensus document on the contested subject.
The […]
CX Peripheral Live and Edited Cases: Why a technology is chosen for a particular situation
cxsymposium2019-09-09T11:03:39+01:00
As part of this year’s Peripheral Arterial Main Programme, Charing Cross yesterday hosted a series of live cases from Münster, Germany. There were also a number of edited cases presented by Faculty from across Europe.
The session was chaired by Michael Jaff (Boston, USA), and moderated by Giovanni […]
Good day for drug-coated balloons, swirling flow stents and drug-eluting stents at CX
cxsymposium2019-09-09T11:04:34+01:00
Yesterday’s Peripheral Arterial Challenges session saw a host of new developments being presented and a lift for drug-coated balloons (DCBs), swirling flow stents and drug-eluting stents.
The day began by trying to avoid any intervention by exercise, best medical treatment and smoking cessation. Presenters showed that […]
Early data from venous stent trials “very promising”
cxsymposium2019-09-10T09:08:46+01:00 Lowell Kabnick
Lowell Kabnick
Two presentations yesterday in the Venous Challenges Main Programme session have demonstrated the value of dedicated venous stents, with interim results from two studies in patients with symptomatic iliofemoral venous outflow obstruction showing symptom improvement 12-months post stent placement.
Feasibility data from the VIRTUS study were […]
CX puts spotlight on vascular and endovascular practice in Latin America
cxsymposium2019-09-10T09:09:42+01:00
The fourth CX Meets Latin America session took place yesterday, highlighting vascular and endovascular techniques used in Latin America. With time for audience discussion following every talk, the session covered not only the technical aspects of different interventional solutions, but the economic context behind them.
“For neurogenic thoracic […]
Attendees debate optimal treatments for “challenging” cases at Vascular Malformations session
cxsymposium2019-09-10T09:14:00+01:00
Yesterday’s Vascular Malformations course began with a talk by Andreas Saleh (Munich, Germany) on four basic principles for diagnostic imaging. According to his first principle, clinicians should make sure to take a thorough patient history, and perform clinical examinations and colour-coded duplex sonography. Saleh then advised that […]
“None of us have the full range of skills and experience” to deal with paediatric vascular pathologies, session finds
cxsymposium2019-09-10T09:14:57+01:00
Yesterday’s CX Paediatric Vascular Issues session tackled some of the specific implications for vascular surgery on paediatric patients. The session was dominated by a discussion of the challenges presented by the different vascular pathologies of children and adults. Faculty and audience members raised […]
Non-thrombotic iliac vein lesions trigger “paradigm shift”
cxsymposium2019-09-10T09:57:02+01:00Now that non-thrombotic iliac vein lesions (NIVLs) are more frequently recognised on modern imaging modalities, they are beginning to trigger a paradigm shift in standard vein work whereby there is now increasing emphasis on making sure that these patients do not have significant deep venous obstruction which must be taken into account when planning treatment. […]
Call for Paediatric Vascular Case submissions
cxsymposium2016-04-21T09:32:28+01:00CX is looking for paediatric vascular management dilemmas to encourage audience discussion at the CX Paediatric Vascular Issues course.
Clinicians are invited to submit paediatric cases concerning acute or chronic limb ischaemia, trauma, mid-aortic syndrome, cancer resection and vascular malformations.
Format:
Cases should be submitted in Powerpoint (maximum three slides or the equivalent to a three-minute presentation). The material should be limited […]
Why you should not miss the Peripheral Arterial sessions at CX 2016
cxsymposium2019-09-10T15:50:05+01:00“The Peripheral Arterial Programme at the Charing Cross Symposium in 2016 is really exciting; it combines the hot topics in the field with evidence, opinion and discussion,” says Andrew Holden (Auckland, New Zealand), member of the CX Programme Organising Board. He overviews key areas to be discussed on Tuesday 26 April including the management of […]
Why you should not miss the Venous Programme at CX 2016
cxsymposium2016-10-12T13:01:12+01:00In 2016, the Charing Cross Venous Programme will run on the four days of the Symposium (26–29 April) with the Main Programme for the first time on day 1, followed by the CX Venous Workshop on days 2 and 3, and the CX Venous Abstract Presentations on day 4. Ian Franklin (London, UK), member of […]
Stephen Black discusses the highlights of the CX 2016 Deep Venous programme
cxsymposium2020-01-02T10:52:04+00:00Stephen Black (London, UK), member of the CX Programme Organising Board, discusses the highlights of this year’s Deep Venous section of the CX Venous Challenges Day.
Charing Cross launches new CX Vascular Access Course
cxsymposium2016-10-12T13:01:12+01:00The Charing Cross Symposium is launching the new CX Vascular Access Course to be held at Olympia Grand, London, UK. The three-day course (27–29 April 2016) will provide both experts and those new to the field with invaluable insights into the challenges currently facing haemodialysis vascular access, and the methods used to overcome them. […]
Chairman Roger Greenhalgh highlights the most exciting aortic topics of CX 2016
cxsymposium2016-03-18T19:16:37+00:00“There has never been a more exciting expectation of what we have for this year in the aortic area at CX,” says Roger Greenhalgh, chairman of the CX Programme Organising Board. Results from the Individual Patient Data meta-analysis of the four randomised controlled trials on endovascular aneurysm repair against open repair (EVAR 1, DREAM, OVER […]
The Charing Cross Symposium introduces a new session on Acute Stroke Challenges
cxsymposium2016-10-12T13:01:12+01:00In 2016, the Charing Cross Symposium will offer delegates a new session dedicated to exploring the challenges in acute stroke treatment. The half-day session will take place on Friday 29 April at Olympia Grand, London, UK.

Discussing the rationale for incorporating this session at this year’s Symposium, […]
Insights into acute stroke challenges
cxsymposium2016-10-12T13:01:12+01:00Ross Naylor (Leicester, UK), member of the CX Programme Organising Board, discusses the biggest challenges of treating acute stroke and the role of a multidisciplinary team in this setting.
 Ross Naylor
Ross Naylor
What do you think are the biggest challenges treating acute stroke?
Improving patient awareness about the need to […]
Long-term data needed for deep venous disease treatment
cxsymposium2016-10-12T13:01:14+01:00Stephen Black (London, UK), member of the CX Programme Organising Board, speaks about the current challenges in the treatment of deep venous disease, key developments in the field, and factors influencing outcomes in venous stenting.
 Stephen Black
Stephen Black
What are the major challenges treating deep venous disease?
We need to […]
Superficial venous treatment: techniques and applications
cxsymposium2016-10-12T13:01:14+01:00Mark Whiteley (London, UK), member of the CX Programme Organising Board, discusses various techniques for superficial venous disease treatment and their applications to specific patients. The latest evidence on this area will be discussed at the CX Venous Challenges Day (26 April 2016).
 Mark Whiteley
Mark Whiteley
Could […]
Explore venous technologies and techniques in the CX Venous Workshop
cxsymposium2016-10-12T13:01:14+01:00The CX Venous Workshop consists of one-to-one demonstrations by world-leading experts and expands on the technical aspects of key superficial and deep venous topics discussed in the CX Venous Main Programme. This year, a new section on aesthetic phlebology will be included in the workshop.

CX Venous Challenges session to explore when to use and when not to use technologies in venous disease treatment
cxsymposium2016-10-12T13:01:15+01:00This year, the CX Venous Challenges Programme will explore the use of various technologies and techniques for the treatment of superficial and deep venous disease, with emphasis on the latest evidence of when and in which patients they should be used. Ian Franklin (London, UK), member of the CX Programme Organising Board, notes: […]
Management of the superficial femoral artery at the centre of the discussions at CX
cxsymposium2016-10-12T13:01:15+01:00
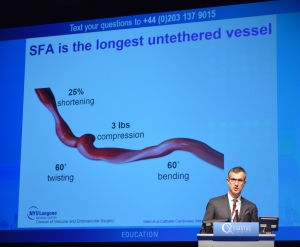
The management of the superficial femoral artery be at the centre of the discussions at the CX Peripheral Arterial Challenges Day of the Charing Cross Symposium 2016. Treatment strategies, depending on lesion type and length, will be analysed and there will be special emphasis on the status and […]
Delegates to learn techniques for femoropopliteal lesions with the CX live-case method
cxsymposium2016-10-12T13:01:15+01:00 CX 2015 Peripheral Arterial Live Cases.
CX 2015 Peripheral Arterial Live Cases.
Following the successful first year of the CX Peripheral Arterial Live Cases, CX 2016 will continue to offer delegates the opportunity to expand the discussion of key topics from the CX Peripheral Arterial Main Programme by learning techniques of how to […]
CX ilegx Collaboration Day addresses proactive approach to treat diabetic foot
cxsymposium2016-10-12T13:01:15+01:00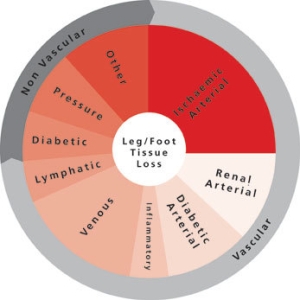
With the aim to promote the best possible care of the ischaemic lower limb to reduce the number of major amputations, the CX ilegx Collaboration Day will offer delegates an overview of the latest treatment strategies, particularly in patients with diabetes, one of the main […]
Mini-symposium explores false lumen challenges in acute and chronic type B dissection
cxsymposium2016-10-12T13:01:15+01:00 Type B dissection.
Type B dissection.
It is accepted that the prognosis and outcomes of type B dissection are more favourable if the false lumen is thrombosed. According to Roger Greenhalgh, chairman of the CX Programme Organising Board, this has been taken by some vascular specialists to imply that the false […]
IPD meta-analysis to show “highest level of evidence” for EVAR vs. open repair
cxsymposium2016-10-12T13:01:15+01:00 Principal investigators: Roger Greenhalgh, Jan Blankensteijn, Jean-Pierre Becquemin and Frank Lederle.
Principal investigators: Roger Greenhalgh, Jan Blankensteijn, Jean-Pierre Becquemin and Frank Lederle.
An Individual Patient Data (IPD) meta-analysis of the randomised controlled trials—EVAR 1, DREAM, ACE and OVER—will show the “highest level of evidence” of endovascular aneurysm repair against open repair for abdominal aortic aneurysms, according to Roger Greenhalgh, […]
Longest follow-up for EVAR to be presented for the first time at CX 2016
cxsymposium2016-10-12T13:01:15+01:00 Roger Greenhalgh.
Roger Greenhalgh.
Fifteen-year follow-up of the Endovascular Aneurysm Repair (EVAR) 1 Trial will be presented for the first time at CX 2016. The EVAR 1 Trial was the first EVAR vs. open repair abdominal aortic aneurysm trial to be conducted and is the first to reach 15 […]
Interview with Michel Makaroun at CX 2015
cxsymposium2016-10-12T13:01:16+01:00Interview with Matt Thompson at CX 2015: EndoVascular Aneurysm Sealing
cxsymposium2015-08-19T11:42:00+01:00Interview with Ian Franklin at CX 2015: New venous technologies
cxsymposium2015-12-18T16:12:28+00:00Ian Franklin, a member of the CX Programme Organising Board and director of the CX Venous Workshop, speaks about new technologies for the treatment of varicose veins and about how deep venous interventions have been incorporated to the CX Venous Workshop.
Interview with Janet Powell at CX 2015: IMPROVE trial 12-month results
cxsymposium2015-08-07T15:50:00+01:00Janet Powell, Imperial College, London, UK, is interviewed about the 12-month results of the IMPROVE trial, presented for the first time at CX 2015. The study compares open repair and an endovascular strategy for ruptured abdominal aortic aneurysms.
GORE TAG Thoracic Branch Endoprosthesis – Interview with Michael Dake
cxsymposium2015-08-07T15:49:46+01:00Colin Bicknell, London, UK, interviews Michael Dake, Stanford, USA, discussing the use of, and early results for, the new GORE TAG Thoracic Branch Endoprosthesis device.
Reconstruction of the ascending aorta – Interview with Rodney White
cxsymposium2019-09-25T09:54:04+01:00Reconstruction of the ascending aorta – Interview with Rodney White
Interview for CX 2015
CX Abstract Sessions – Interview with Richard Gibbs
cxsymposium2015-08-07T15:49:00+01:00Richard Gibbs, member of the CX Abstract Board, speaks about the 2015 CX Abstract Sessions, their importance for this year’s Charing Cross Symposium, and how these sessions can help spotting new talent.
CX Imaging Day provides unique view
cxsymposium2016-10-12T13:01:16+01:00The CX Imaging Day provided a comprehensive programme of state-of-the-art imaging that is applied to the aorta, carotid, peripheral arteries and veins, including planning and using hybrid theatres, robotics, 3D fusion and guidance, intravascular ultrasound, perfusion angiography, cone beam computed tomography and carbon dioxide angiography.
This year’s Imaging Day was divided into two parts, giving attendees the opportunity for maximum learning and understanding. The morning session saw the presentation of the latest data in the Olympia Room Learning Centre, while in the afternoon, various imaging providers, including Philips, GE Healthcare, Siemens, Hansen Medical, Volcano and Ziehm Imaging, hosted “Ask the Expert” workshops at their stands in the Exhibition Hall. These workshops allow attendees to interact in small groups with the experts and to look at the imaging systems and tools, providing them with a unique view of the technology.
GE Healthcare
GE Healthcare took the opportunity to show the flow of its solutions from sizing to the 3D fusion from table side. There was also focus on dose optimisation management.
Alejandra Gonzalez, European marketing product manager for Interventional Systems at GE explained, “We provided an educational framework where we raised awareness of the key elements that physicians need to take care of in order to reduce the dose or optimise the dose strategy. We wanted to do it in an educational way and at the same time a more interactive way as well.”
Guiding the presentations and providing personal input were physician experts Stephan Haulon (Lille, France) and Adrien Hertault (Lille, France). Haulon explained what took place saying, “we showed the attendees a couple of cases that were sized on a workstation so they had the ability to actually manipulate the workstation and then we had them do a fusion registration to show them how straightforward a technology it is and how user friendly it is. We also had a big focus on dose exposure which is a real concern for vascular surgeons today because we are doing more and more radiation exposure procedures. We had a quiz which stirred a lot of questions and debates. So this was actually quite passionate and really interesting.”
Commenting on the workshop Haulon added, “There was a lot of interaction and those people would never stand up in a large room to ask a question, whereas here they were very enthusiastic about sharing their comments and asking us for more details so I think it was a very valuable workshop.”
Hansen Medical
Hansen Medical presented the Magellan robotic system which helps physicians navigate through the vasculature. Joe Guido, vice president marketing and business development explained that the system is designed to “make the procedure more efficient and more predictable for physicians and also allows physicians to stay out of the field of radiation. The robotic system will drive robotic catheters that you can shape and control without having to do exchanges for other catheters.”
Physician experts Barry Katzen (Miami, USA) and Celia Riga (London, UK) presented their experiences with intravascular robotics and the Magellan robotic system.
Guido commented on the workshop saying that many physicians are very curious about the technology and the practical applications and how it can benefit them and their practice. “An expert is much more accessible at the stand and it is a much more intimate environment. It encourages physicians to listen to the presentation and then speak to the experts and ask one-on-one questions,” he said.
Philips
Philips presented its technology and had physician experts Frank Vermassen (Ghent, Belgium) and Jim Reekers (Amsterdam, Netherlands) on hand to answer queries from physician-attendees. Philips demonstrators showcased the company’s technology including the VesselNavigator, a live image guidance solution; 2D Perfusion; Veradius Unity, a C-arm with flat detector; and gave advice on considerations when purchasing a hybrid room.
Commenting on the Veradius Unity, Philips demonstrator Marianne Kwakernaat said that the technology was developed, “to help get a more united team in the OR because there was a lot of frustration between doctors and operators who did not understand each other, so we made a lot of improvements to our systems to first, make it more easy to communicate, so we added some tools, and second, we made a new touchscreen which is very interactive and easy to use.”
She added that the workshop was a good vehicle to give the physicians the opportunity to see the products and figure out which one is most suitable to their hospital and their uses.
Siemens
Siemens showcased the complete workflow for EVAR procedures, starting with the preoperational CT scan. The company also gave attendees the opportunity to see the PURE software technology for the Artis zeego.
Dirk Sunderbrink, business manager, Therapy Systems at Siemens described the system saying, “It allows the vascular surgeon 2D and 3D registration with much less dose and in much less time than with previous systems. This PURE software guides the vascular surgeon through the procedure automatically to have fusion imaging available to guide them through the procedures.” He added, “The teaching opportunity is important to us because there are presentations, but it is much easier to really see it hands-on.”
Dittmar Böckler (Heidelberg, Germany) was on hand providing an expert position, giving attendees the opportunity to ask questions about the Siemens technology.
Volcano
Volcano showcased its intravascular ultrasound (IVUS) system, which provides detailed and accurate measurements of lumen and vessel size, plaque area and volume, and the location of key anatomical landmarks.
Physician expert Fabrizio Fanelli (Rome, Italy) was present to give his personal perspective of the technology and to answer attendees’ queries. At the stand, demonstrations were done showing real time sizing and procedure assessment with reduced exposure and contrast.
Volcano demonstrator Fiorella De Nicolais explained that the IVUS technology helps differentiate the four plaque types: fibrous, fibro-fatty, necrotic core and dense calcium.
She added that, “Not everyone knows IVUS, or the way it works or the benefits of using it, so the workshop is about getting more details and learning how the technology works.”
Ziehm Imaging
Ziehm Imaging featured its mobile hybrid room concept which consists of a mobile C-arm that has all the imaging capabilities of a fixed, installed hybrid room—high power imaging, a liquid cooling system, flat panel technology, fully motorised movements.
Visitors to the stand heard presentations from experts Wolfgang Keller (Nuremberg, Germany) and Peter Goverde (Antwerp, Belgium), who spoke about Ziehm’s mobile interventional suite and their clinical experiences with a mobile hybrid OR.
Summarising, Axel Kouril from Ziehm Imaging said, “As opposed to fixed installed hybrid ORs which are very expensive and have additional installations costs of enforcing the floor or the ceiling, and lead protection, this system has the size of a normal mobile C-arm and the imaging capabilities of a fixed installed system (up to 90%), but it is cheaper, it is mobile, and more flexible.”
A record-breaking week for the CX Venous Workshop
cxsymposium2016-10-12T13:01:16+01:00The second day of the CX Venous Workshop was held yesterday on the Upper Level of the Gallery, rounding off a busy week in which more than 950 people visited over the two days—a new record for the Workshop. Once again the educational stations, of which there were more than 30, were packed with delegates looking forward to one-on-one time with the distinguished Charing Cross Symposium faculty.
As was the case on Wednesday, the “Endovenous Ablation Village” was running, showcasing all available techniques to ablate truncal veins, along with practical training sessions teaching ultrasound-guided cannulation, catheter positioning and tumescent anaesthesia.
While the first day of the workshop focused largely on varicose veins and superficial venous issues, yesterday went deeper into the vein by examining acute deep vein thrombosis, intravascular ultrasound and deep venous stenting. Every station saw a significant amount of traffic, with delegates showing a keen interest in the intravascular ultrasound and deep vein thrombosis stations.
Ian Franklin, one of the course directors, said “Today we have had a big change of interest to deep venous—treatments of deep vein thrombosis, deep vein stenting, intravascular ultrasound, pelvic vein embolisation and so on—and it is packed. There is not a single empty training station, everyone has good interest and it is sustained. We deliberately have this flexible format so people can come and go—if they find they have a gap in the programme they can come and join us and make sure their time is used profitably.”
As the workshop drew to a close for another year, thoughts turned towards 2016. “Every year we change the event and it has never been the same as it was before, so next year will definitely be different,” said Franklin. “One thing I would consider is bringing in recorded live cases of all the new techniques we are showcasing to complement the hands-on demonstrations, using screens and some commentaries.”
With the workshop in its seventh year, there is always the opportunity to resurrect popular aspects of previous years’ Workshops. For example, Franklin suggested, “I would also like to bring back interactive discussion of complicated and difficult cases. That was always very popular, especially when you had a series of well-known experts all discussing how best to handle a case. Quite often we are showcasing different techniques and then once you have learnt that technique there comes the discussion of when to use it, so I think those case-based discussions complement the learning too.”
Day 2 offered stations covering: thermal (laser), thermal (radiofrequency ablation), non-thermal, practical training stations, valves, vascular ultrasound training simulator, venous malformations, pelvic venous imaging, pelvic vein embolisation, acute deep vein thrombosis, caval filters, follow-up protocols, air plethysmography, intravenous ultrasound and deep vein stenting.
Alma Lasers was exhibiting its VascuLife minimally invasive varicose vein laser treatment with robotic pullback. Having received CE mark and FDA approval recently, the VascuLife station had plenty of visitors looking to learn about the robotic pullback capability, which standardises pullback rate safely and allows a surgeon to focus on other tasks while pullback is completed. Shira Doron, marketing director at Alma Lasers, believes that the CX Venous Workshop gave companies and physicians a chance to interact and share information in an informal and purely scientific setting. She said, “Physicians view this as their professional platform as opposed to booths, which may feel like more of a marketing platform. They come here and it is all about the device and what it can do. You do not need to see movies or brochures—it is all about the technology.”
The new ArtVentive EOS device also was highlighted yesterday. Krzysztof Pyra (Lublin, Poland) spoke his experience with the EOS device treating ovarian veins for pelvic congestion syndrome. In addition, treatment of spermatic vein varicoceles were also presented. Pyra discussed his approach to diagnosing and treating pelvic vein incompetence. According to Pyra, of particular note was the immediacy of occlusion with the EOS device and shorter procedure times. Pyra uses a combination of EOS and sclerosant to occlude the pelvic veins.
Early results with a new thoracic branched device for the arch “are promising”
cxsymposium2015-07-11T02:23:22+01:00Michael Dake, Stanford, USA, presented the initial results with the GORE TAG Thoracic Branch Endoprosthesis (TBE), a new device for treatment of the aortic arch at CX yesterday.
Dake, Stanford, USA, presented the initial results with the GORE TAG Thoracic Branch Endoprosthesis (TBE), a new device for treatment of the aortic arch.
Dake told delegates that the GORE TAG TBE is a single branch device that consists of an aortic component, a side branch component and an optional aortic extender. The side branch component is delivered through a sheath and docks into the aortic component portal which allows for perfusion of a single arch branch vessel. The device is currently being studied in a zone 2 (left subclavian) feasibility study and a zone 0/1 (brachiocephalic and left common carotid) early feasibility study in the USA.
Currently, there are 20 patients (10 men and 10 women, mean age 75.4 years) successfully enrolled in zone 2 and one patient enrolled in zone 0/1. All 20 patients in zone 2 had successful access and deployment of the TBE device with the side branch component patent at the end of the procedure. Additionally, all side branch components remain patent. At one month, there have been no reported patient deaths or stroke. The left ankle brachial index (1.1) remains the same from pre-procedure to one month. There were no endoleaks that required reintervention.
The first two zone 0 patients were treated with the TBE device at the University of Pittsburgh Medical Center by Michel Makaroun, and at the Mayo Clinic by Gustavo Oderich. The protocol requires the patient to be treated in two phases with phase 1 revascularisation and phase 2 endovascular procedure. In phase 1, the patient underwent cervical debranching of the arch via a carotid to carotid bypass followed by a left carotid to left subclavian transposition. The proximal left common carotid and left subclavian artery was suture ligated.
Dake spoke about the patient treated about Makaroun and said the patient tolerated the procedure well without any neurological complications. The patient was assessed and deemed stable to proceed to phase 2 after 24 hours. In phase 2, the aortic component was advanced and deployed at the target location near the brachiocephalic artery. The sheath was advanced without difficulty into the portal of the aortic component into the brachiocephalic artery. The side branch component was advanced and deployed. Final arteriography showed excellent flow into the arch vessels, aneurysm exclusion, and complete apposition of the aortic component both proximally and distally.
Dake concluded: “These early results are promising with 100% technical success and side branch patency along with 0% death or stroke at one month.”
Fear of being sued may force healthcare providers into improving access to diabetic foot care services
cxsymposium2015-07-11T02:23:22+01:00Although there is strong evidence that multidisciplinary foot care services can reduce the number of amputations associated with diabetic foot, access to such services remains patchy. Cliff Shearman (Southampton, UK) believes that healthcare providers are not doing enough to improve access. He says that it may take litigation from patients, who have lost a foot through poor care, to persuade healthcare providers to increase access, which he says would be “a great pity” because it should be “evidence and enthusiasm” that drives change.
Speaking at the CX ilegx Collaboration Day yesterday, Shearman explained that early diagnosis and prompt treatment can help to reduce the risk of amputation in patients with diabetic foot. He added that there is “strong evidence” to show that well-organised diabetic foot teams, working across primary and secondary care, can ensure that patients receive the care they need. Shearman noted that research indicates that such teams can the reduce rate of amputation by up to 80%. Furthermore, he reported that having a foot care team at his hospital (Southampton) has resulted in costs savings of £1,695,600 and a reduction in total beds of 5,662.
However, in the UK, only around 50% of people with diabetes receive the recommended nine health checks, including foot examination, and up to one in five hospitals do not have a diabetic foot protection team. Shearman commented: “I think healthcare professionals are now much more interested in the need to provide good care to reduce the amputation rates, but there is a lack of drive from government and healthcare providers to push this forward. At the moment, in the UK, we do not have core criteria for commissioning diabetic foot care services—all we can really do is to try to emulate those who have created good services,” he said.
According to Shearman, there are three steps to encouraging healthcare providers to take steps to increase access to foot care services: enthusiasm and evidence, naming and shaming, and litigation. He claimed that we are now the stage of “naming and shaming”—ie, showing how amputations rates can be “staggeringly reduced” with timely access and multidisciplinary team working. “If that does not work, it will be litigation that drives change. The number of ligation cases is rising enormously because people are recognising that they have not received good care and therefore, are taking action against their healthcare provider. It will be a great pity if litigation is what prompts change as it should be enthusiasm and evidence.”
Michael Edmonds (London, UK), who (like Shearman) is a course director of the CX ilegx Collaboration Day, agrees that poorly organised services—rather than a lack of effective treatment—are a cause of patients with diabetic foot having to undergo amputation. He said one of the key goals of ilegx, which was started in 2008, was to “encourage interdisciplinary collaboration that spans the primary and secondary services, and develop and implement best practice approach to save legs.”
Shearman told CX Daily News: “I hope delegates who attend the course will go away fired up with the knowledge that they can make a difference [by being enthusiastic about improving care and encouraging multidisciplinary working] and that they can help their centres save money as well as improve outcomes. Improving the care of patients with diabetic foot is not about hiring lots of new people or lots of new technology. It is about working as a team.”
Treatment options for diabetic foot
During the CX ilegx Collaboration Day, there were several talks about treatment options for patients with diabetic foot. Toby Richards (London, UK) spoke about a novel wound therapy called CelluTome (KCI). He said that this involved taking an epidermal graft off a patient’s leg, without any form of anaesthetic, by applying local heat to create microblisters that were then transferred onto a wound.
To assess the system, Richards and colleagues evaluated its use in a case series of 30 patients who were candidates for split skin grafts—most of whom had chronic wounds, with Richards noting: “so, we are not picking winners” (ie. patients with wounds that had a better chance of complete healing).
He stated that the “most important thing” was that the average time for donor site healing was 5.43±1.54 days, the mean pain score was 1.33±0.95, and the Vancouver Scar Scale was 0 for all cases at six weeks after therapy.
Richards reported that 17 patients had complete healing (12 in under six week and four in under eight weeks) and that seven grafts failed due to infection, adding “one of the problems we had is that the graft looks like a biofilm at two weeks and a nurse who was not familiar would come along and think it needed cleaning, so there goes your graft. While you get used to this look, you need to leave the dressing on as long as possible, and then you will start to get good results.” He concluded that it was a novel technology that was “feasible” and it that “certainly does work”, saying: “as with all new technologies, it should be tested in a proper trial before we introduce it into normal practice. Therefore, we will be evaluating it in a randomised controlled trial.”
Jennifer Tremlett (London, UK) also spoke about wound care techniques, including platelet rich plasma, larvae therapy, and negative wound pressure. Like Shearman and Edmonds, Tremlett advocated “multidisciplinary working” because it “provides better outcomes”. Furthermore, Dean Huang (London, UK), who spoke about advances in distal endovascular surgery, said that the need for collaborative working and rapid access to diagnosis and intervention were “probably two of the most important points” in his talk.
“We can solve the limitations of carotid stenting with a micro-mesh stent and its sustained anti-embolic action”
cxsymposium2016-10-12T13:01:16+01:00Micro-mesh stents and sustained anti-embolic action to reduce cerebral embolisation may contribute to solving the remaining limitations of carotid stenting, according to Alberto Cremonesi (Cotignola, Italy), who spoke at CX 2015 (28 April–1 May, London, UK).
Cremonesi examined the potential for micro-mesh stents to improve existing carotid artery stenting outcomes.
He told delegates that the correct technique for performing a carotid artery stenting was to conduct a pre-procedural evaluation for common carotid engagement, to select the correct stent and to select and manage an embolic protection device.
Cremonesi said that it is already known that “not all plaques are the same”, and that “carotid artery stenting and carotid endarterectomy are —and will remain—emboli-generating procedures”. He told the audience that it is possible to give sustained anti-embolic over time using specific stents.
When a poorly-selected stent is used for carotid artery stenting, intra-strut prolapse can occur resulting in post-procedural embolic events. The difficulty then, commented Cremonesi, is that “lesions in the carotid arteries are often anatomically and morphologically very challenging. For this reason, plaque coverage can play an important role.”
The use of stenting strategies such as open cell, closed cell and hybrid geometry can be considered “time honoured”. Now, vascular specialists have access micro-mesh double layer carotid stents (Terumo, InspireMD and Gore), said Cremonesi, whose personal experience is with the Roadsaver carotid stent from Terumo.
Cremonesi admitted that “In terms of data, we do not have very much at the moment” for the use of micro-mesh. Some data do exist from the CARENET all-comer trial, in which 30 patients saw “very good” outcomes without any neurological events up to five months follow-up. A further multicentre trial with 100 patients, CLEAR-ROAD, is also beginning, though Cremonesi said that he did “not want to conclude anything” about it yet.
“We are probably changing the paradigms in carotid artery stenting, because with these micro-mesh stents and their sustained anti-embolic action, we can solve the remaining limitation of carotid stenting,” ended Cremonesi.
Discussing his presentation with the audience, Cremonesi mentioned that “two-thirds of the embolic complications are in the post-procedural phase.” When session chairman Roger Greenhalgh asked about the cause for this later onset of complications, Cremonesi told him that it was due to the stent interacting with and cutting the plaque in the carotid artery, which can occur during the phase and even up to 48 hours later. Cremonesi made it clear that this problem is one of the carotid artery, rather than one associated with the aortic arch.
Despite endovascular revolution, 65% say heyday of open repair in aortic arch continues
cxsymposium2016-10-12T13:01:16+01:00There have been vast technological advances that have enabled the final frontier of the aorta, the diseased ascending aorta and arch, to be treated by completely endovascular means. Simultaneously, there have also been advances in open repair. Also, with perioperative stroke remaining a principal risk with TEVAR, the high incidence of cerebral embolisation with the procedure is a problem that needs wider recognition, delegates heard yesterday.
In a panel discussion during the session on interventions for ascending aorta and aortic arch, Roger Greenhalgh, chairman of the CX Organising Board, pressed the panel to comment on patient mortality after open repair and endovascular repair.
The mortality rate for open repair is around 3%, even when there is involvement of the aortic valve, said Stephen Large, Cambridge, UK. With endovascular repair, it approaches 10%, said Dittmar Böckler, Heidelberg, Germany. Subsequently, 65% of the CX 2015 audience voted against the motion that the heyday of open aortic surgery is over.
Richard Gibbs, London, UK, told CX delegates yesterday that there was a high rate of embolisation during arch and descending thoracic intervention that could be observed as silent cerebral infarction on new imaging techniques. The results from the study he presented showed that there is a 70% silent cerebral infarction on MRI and that there is postoperative neurocognitive decline in patients with silent cerebral infarctions.
Gibbs made the point that stroke, which was caused by cerebral embolisation, was a relatively crude surrogate, but a clinically relevant measure of microembolisation. The stroke rate for TEVAR ranges between 3% and 6%, said Gibbs, with embolisation being caused by the passage of stiff wires, soft wires, devices and manipulation within the diseased aorta. The risk factors are the atheromatous burden within the aorta and using a proximal landing zone. Hypotension is also very important, he said.
“A much subtler way of looking at microembolisation is with diffusion-weighted MR that detects acute ischaemia that is due to cerebral oedema. These lesions appear within 24 hours of the insult and last up to 14 days, and show as hyperintense bright areas that are easy to recognise,” Gibbs noted.
Gibbs then showed diffusion-weighted cerebral MR images from a patient who had silent cerebral infarctions but did not develop signs or symptoms of clinical stroke following TEVAR. “Therefore what we see here is silent stroke, or silent cerebral infarction, which is imaging evidence of cerebral infarction, without a history of acute neurological dysfunction attributable to the lesion,” Gibbs said.
He qualified that he believed the term silent cerebral infarction is a misnomer because there is an increasing body of evidence that suggests that silent cerebral infarction is associated with depression, dementia, Alzheimer’s, future increased risk of stroke and mortality.
Published literature shows a significant rate of cerebral infarction with various different interventions involving the arch and unsurprisingly transcatheter aortic valve implantation (TAVI), which is the biggest device and causes the most cerebral infarction, he explained.
“When it comes to TEVAR, there is very little literature, and one paper suggests that there is a 63% risk of cerebral infarction with the procedure,” said Gibbs referring to the data from 19 patients from Kahlert et al published in the Annals of Thoracic Surgery in 2014 that found diffusion-weighted MR evidence of cerebral infarction after TEVAR in 12 of 19 (63%) undergoing the procedure for a variety of indications. There was no overt clinical stroke seen in these patients.
Gibbs then presented the pilot work done by his team on the rates of silent cerebral infarctions during TEVAR, the presence of silent cerebral infarction on MR and whether there are neurocognitive changes afterwords.
“We included 44 patients undergoing TEVAR and looked at the burden of atheroma within the arch of the descending aorta based on the American Heart Association grading method. The patients had bidirectional transcranial Doppler, which is a direct measure of cerebal microembolisation. “A subset of these patients had pre-and postoperative MR looking for more evidence of infarction (23 patients). Another subset had neurocognitive assessment before the intervention, after the intervention in hospital and eight weeks later to see if any changes persist,” said Gibbs.
Forty one TEVAR procedures involving the arch and descending aorta were performed for a variety of pathologies. Of these 21 were standard and 20 were complex involving the use of branches, scallops or adjunctive surgical procedures. “We had fairly proximal landing zones,” he noted.
“Looking at the specific procedural steps, we see clearly that stent graft deployment carries the most significant rate of embolisation,” said Gibbs. He then showed a transcranial Doppler during stent graft deployment where a burst of cerebral embolisation was visible in both hemispheres. “The highest activity of cerebral embolisation was when the device was deployed. Increased embolisation was associated with the left hemisphere more than the right; stent manipulation more than wire and catheter passage; a higher grade of atheroma compared to a lower grade (so a higher burden of disease); chronic rather than acute disease. Patients who had a stroke had the highest rate of embolisation. A proximal landing zone rather than distal one had a higher rate of cerebral embolisation. In the group where we looked for evidence of silent cerebral infarction, 70% (16/23) of our patients had this, mostly in the left hemisphere and mostly in the territory served by the middle cerebral artery. Ten per cent of these patients had a clinical stroke,” said Gibbs.
When the researchers selected out the data for older patients from the group, they found that executive function diminished and stayed down at eight weeks, memory diminished and manual dexterity got worse.
“There is a significantly high rate of cerebral embolisation during TEVAR and the more proximal you go, the worse it is. There is a definitive and radiologically proven damage to the brain and these patients do pay a price for this. We have to be thinking about how we can intervene [either pharmacologically or by using devices],” concluded Gibbs.
Scarcity of literature on proximal landing zone and TEVAR outcomes
Dittmar Böckler stated that while the relationship between the proximal landing zone and outcome was well-documented in the EVAR literature, there were very few publications focused on how the proximal landing zone influences patient outcomes with TEVAR in the arch, and noted the low level of evidence.
“There are no randomised controlled trial data on open versus endovascular approaches and there are no meta-analyses. The data come from limited European registry data from the Relay registry, Traviata registry and European CTAG registry,” he noted.
Böckler drew attention to the fact that there was no consensus in the literature and guidelines on the appropriate proximal neck length and that this variation was reflected in the instructions for use from manufacturers, as the target landing zone is depending on stent graft diameter.
“The appropriate proximal landing zone for TEVAR is not defined. Arch type and atheroma seem to influence stroke risk during TEVAR in the arch. “There is strong need for new refined conformable devices in the arch including branched stent graft technology,” Böckler said.
Value of medication
Frank Lederle, Minneapolis, USA, speaking on the value of medication such as statins before aortic arch catheterisation, stated that there was very little trustworthy randomised controlled trial data to go by. He reviewed evidence that bears on whether perioperative statins or aspirin benefit patients having a thoracic aortic procedure. “Analyses showing benefit of perioperative use rely on weak and doubtful studies. When it comes to aspirin, there are no data on perioperative benefit, but there is an increased major and minor bleeding,” he said.
Lederle drew attention to the fact that many patients with aortic aneurysm have arteriosclerotic cardiovascular disease and should be on statins and aspirin long-term. “There is no good evidence that statins or aspirin improve long-term outcomes for patients with aortic arch patients without arteriosclerotic cardiovascular disease, so some of these patients will not be on these medications. Questions remain on when whether or not patients are on them long-term and whether they should be used perioperatively,” he said.
Open surgery
Stephen Large, Cambridge, UK, a cardiac surgeon, outlined the case for open surgery in interventions for ascending and arch of the aorta, the current gold standard approach.
Large noted that not operating resulted in a “dreadful attrition”. “We know that there is an increase in attrition correlated with the increase in aneurysm size, the hingepoint being around 5.5cm in the ascending and arch, which is associated with an acceleration in terms of stroke, dissection rupture and death,” he said.
“What we very often do in the ascending aorta is deal with an associated post-aortic valvular lesion either by an interposition graft, that is something placed above the coronary artery ostia, really at the level of the sinotubular junction, and right up to the origin of the innominate artery—a true ascending aortic replacement. We can, if we are in trouble (of course with involvement of pathology within the aortic root), replace the aortic root and that always requires reimplantation of the coronary ostia, which brings a raft of problems of threatening ischaemia with it. Up until relatively recently, this involved automatic replacement of the aortic valve. There is now a keen interest in considering valve preservation procedures, something that I have fought against for many years, because the aortic valve is embryologically of the same origin as the ascending aorta. However, counterintuitively, it appears that the aortic valve fares very well. So what to do with the arch? We can replace it with a tube graft and address each of the usual three arch vessels. Or we can translocate the whole of the aortic arch vessels permitting the use of either replacement or stenting. As cardiac surgeons, we find ourselves replacing the ascending aorta in an emergency as a life-saving procedure for dissection. We, of course, will look at ascending aortic aneurysms for elective surgery for prognostic issues and this is often in conjunction with other procedures in the chest such as aortic valve replacement,” he said.
Endovascular procedures a valid alternative in selected patients
Piergiorgio Cao, Rome, Italy, speaking on branched stent grafts for the treatment of complex arch lesions, said that any repair of aortic arch remains demanding and exposes patients to mortality and stroke risks that are “not negligible”. Open repair is the gold standard, and hybrid and endovascular repair are valid alternatives, mostly in patients who are at high risk for surgery, he noted.
Cao defined the morphological feasibility to receive endovascular treatment as the presence of a proximal landing ≥2cm in length and ≤4.2cm in diameter. The challenges for TEVAR in the arch include conformability of the stent graft, endoleak and retrograde dissection occurence, he said.
He then alluded to a recent publication in the Journal of Vascular Surgery from Paola De Rango et al that analysed total aortic arch reconstruction in a contemporary comparison of current open and endovascular repair.
De Rango et al entered endovascular and open arch procedures performed from 2007 to 2013 into a prospective database and then retrospectively analysed the data. Endovascular repair (proximal landing zones 0-1), with or without a hybrid adjunct, was selected for patients who were sicker but who had a fit anatomy. Operations involving coverage of left subclavian artery only (zone 2 proximal landing) and open hemiarch replacement were excluded.
As reported in the journal, the authors concluded that despite the older age and a higher comorbidity profile in patients with challenging aortic arch disease who were suitable and selected for endovascular arch repair, no significant differences were detected in perioperative and four-year outcomes compared with the younger patients undergoing open arch total repair.
Yesterday, Cao concluded by saying: “The endovascular approach is a valid alternative to open surgery for all patients, when morphologically feasible. A safer proximal landing zone with longer coverage of the ascending aorta may be the key for long-term durability and to prevent retrograde dissection. Branched stent grafts might be useful in avoiding arch manipulations and decreasing the risk of major adverse events,” he said.
Greenhalgh then commented that the audience might like to have some idea of the expected mortality associated with both the open and endovascular methods in the case of a 70-year-old patient with an ascending dilating disease process in which the valve becomes incompetent. Mortality is 3% with open repair and 10% with endovascular repair, the audience learned.
He further commented on the resurgence of the classification of aneurysmal disease: “The starting point seems to be aneurysmal disease. We are seeing comments on syphilitic, fusiform and saccular aneurysms and this is beginning to look like an old surgical textbook, he commented.
In a debate, Hans-Henning Eckstein, Munich, Germany, argued against the motion “The heyday of open aortic surgery is over” to garner majority support that open repair still had a valid place in the treatment of the ascending aorta and aortic arch. He persuaded 65% of the delegates to vote against the motion. Frank Veith, New York, USA, spoke for the motion.
Data evaluating the new Eluvia drug-eluting stent demonstrate 94.4% primary patency rate at nine months
cxsymposium2015-07-11T02:23:22+01:00A clinical trial evaluating Eluvia Drug-Eluting Vascular Stent System (Boston Scientific) has met its primary endpoint with more than 94% of the lesions treated remaining open at nine months post implantation. This was accompanied by a target lesion revascularisation rate of less than 4%.
Results from the MAJESTIC trial were presented yesterday by Stefan Müller-Hülsbeck, Flensburg, Germany, at the CX Abstracts Session – Peripheral Arterial. The trial enrolled 57 patients across Europe, Australia and New Zealand with an average lesion length of 70.8mm.
“I have not seen clinical data this impressive for a vascular stent that has to perform in an environment as challenging as the superficial femoral artery,” said Müller-Hülsbeck. “Because of forces created by knee flexion, there is an increased risk of restenosis, but we are seeing little evidence of this in the MAJESTIC trial.”
The nine-month follow-up showed no deaths or amputations.
The Eluvia Stent System is a stent purpose-built for the superficial femoral artery and uses a polymer and paclitaxel combination designed to facilitate a sustained drug release to reduce restenosis. The system is built on the Innova Stent System platform consisting of a self-expanding nitinol stent with a paclitaxel-eluting biostable polymer matrix loaded on a low-profile delivery system. According to Boston Scientific, the stent architecture features a closed-cell design at each end of the stent for more predictable deployment, and an open-cell design along the stent body for improved flexibility, strength and fracture resistance.
The Eluvia Stent System is pending CE mark and is not available for use or sale in the USA.
CX brings peripheral study data to life with live cases
cxsymposium2015-07-11T02:23:22+01:00Yesterday, for the first time, the Charing Cross International Symposium broadcast peripheral live cases that were directly linked to data presented the previous day on the theme of peripheral arterial controversies. The aim of the course was to provide delegates with opportunities to learn techniques to achieve the best results with the approaches discussed in the data.
Speaking from Bad Krozingen via satellite video, Thomas Zeller (Bad Krozingen, Germany), the director of the peripheral live cases course, said: “The live case transmissions are little bit different from what people are used to seeing at conferences. Our goal is not to educate or teach you about complex interventions; our goal is to provide you with context to data from recently published trials or even ongoing trials.” He explained that he would be the chief operator in the cases and would be assisted by Alijoscha Rastan, Elias Noory, and Ulrich Beschomer (all from Bad Krozingen, Germany).
The first case of the course was related to the 24-month ILLUMENATE data that were presented on Tuesday at CX by Stephan Duda (Berlin, Germany). It involved a patient who was enrolled in the ILLUMENATE Global Registry, which is evaluating Spectranetic’s Stellarex drug-coated balloon. Zeller explained that the patient appeared to have very mild disease of the distal superficial femoral artery. He added that they had purposely chosen a patient with a “relatively simple lesion” to remind the audience that “almost all of the data you can see so far regarding drug-eluting balloons are TASC A or B lesions”.
Zeller reported that he would be using an 8cm drug-coated balloon (with a 4cm balloon to predilate the vessel) because the rule for using drug-coated balloons should be the same as that for using stents in the coronary arteries: treat from a healthy segment into a healthy segment. He explained that the patient in this case had diffuse disease in proximal areas.
According to Zeller, another rule for using drug-coated balloons was not to put the balloon into the vessel until “everything was prepared”. He said: “Preparation means slashing off the guidewire; it is evacuating cracked air from inside the vessel so that you can see the exact actions of the balloon when it is inflated. If you wait until the balloon is inside the vessel to do the preparation, the balloon is already exposed to the bloodstream; the longer the balloon is exposed to the bloodstream, the more drug you will lose from the surface.”
The second case, in which the Silverhawk atherectomy device (Covidien-Medtronic) and a drug-coated balloon were used to treat a popliteal artery stenosis, was also related to data presented during the peripheral arterial controversies. Zeller commented that the rationale for using atherectomy in this patient was based on the results of the DEFINITIVE AR study, which indicated that directional atherectomy and anti-restenotic therapy (DAART) could be used to improve patency in long and severely calcified lesions: the Zeller himself presented the study’s 24-month results at CX on the peripheral day. However, he commented that the patient in the case was not the typical “DEFINITIVE AR” patient because her femoral artery was “free from disease”. “The popliteal artery, in particular the distal segment, is severely exposed to extra compression forces. Therefore, we usually try to avoid placing a stent in this artery. Thus, we used atherectomy before a drug-coated balloon in this particular patient to try to avoid the need for a stent,” Zeller explained.
Rotational atherectomy, with the Jetstream device (Boston Scientific), and a drug-coated balloon (Ranger, also Boston Scientific) were used, in the third case, to treat a calcified superficial femoral artery lesion. According to Noory, who gave the overview of the case, the first-in-man data for the Ranger device were not yet available but data from the preclinical studies were “promising”. The case sparked a discussion about when to use atherectomy and when to use rotational atherectomy, with Zeller commenting: “Rotational atherectomy is a pretty good tool for occlusions. Also small vessels, 4mm for example, also respond very well to the Jetstream system. If you have larger diameters, 5–6mm vessels, or bifurcations then I prefer to use atherectomy.”
The final case of the morning was supposed to involve the use of the TurboHawk (Covidien-Medtronic) and a drug-coated balloon for the treatment of a lesion in the common femoral artery. This related to the ongoing PESTO-CFA study, which is comparing percutaneous intervention with surgery for the management of common femoral artery lesions. However, Zeller had difficulties putting in the guidewire in the lesion and, after discussing the case with the audience, decided to proceed to putting in the drug-coated balloon without using atherectomy.
In the afternoon, cases were focused on situations in which “stents were unavoidable or beneficial” and “where stents are unavoidable or beneficial and in-stent restenosis treatment”. The devices used in these cases were the Zilver PTX (Cook Medical) with a new release system, the Supera (Abbott Vascular), the Supera with a provisional re-entry device, the Innova stent (Boston Scientific), the SmartFlex (Cordis), a sirolimus-eluting balloon and the BioMimics stent (Biosensors), heparin-bonded contoured-edge Viabahn (Gore Medical), and Rotarex (Straub Medical) and IN.PACT Pacific (Medtronic). During Tuesday’s peripheral programme, there were talks reviewing the current controversies in peripheral stenting.
Giovanni Torsello (Münster, Germany), who chaired the morning session of the live cases course, said the live cases were a valuable educational tool because delegates not only wanted to hear about study data but also see “how they can make their strategies, their techniques better than before. This is why people attend live case courses.”
LINC@CX
LINC (Leipzig Interventional Course) once again held a live case session at CX, which this year focused on below-the-knee interventions. The cases included recanalisation of an infrapopliteal obstruction with a drug-coated balloon, retrograde transpedal recanalisation with 3F access system, and lesion-specific use of a drug-eluting stent and a drug-coated balloon for an infrapopliteal obstruction. Giancarlo Biamino (Mercogliano, Italy) told CX Daily News that live cases that “do not go according to plan” were just as educational as the ones that did because it gave delegates opportunities to see how operator can manage and rectify unexpected situations.
70% say going “off-label” to use EVAR in necks less than 10mm in length can be sensible
cxsymposium2015-07-11T02:23:22+01:00In traditional Charing Cross fashion, yesterday’s debates stirred much discussion in the Main Auditorium. Particularly stirring was the debate where the audience voted 70% against the motion that EVAR is not sensible for any abdominal aortic aneurysm with a neck length less than 10mm. Stephen Cheng (Hong Kong) spoke for the motion, while Jan Blankensteijn (Amsterdam, Netherlands) sounded-off against the motion.
Making his case, Cheng said that even with new devices, there are more early complications, more late complications, more (difficult) secondary interventions and more adjuncts associated with short-neck endovascular aneurysm repair.
On the other hand, his opponent maintained that in selected cases of infrarenal neck length <10mm, “Any of the current infra/transrenal devices can be used with or without endoanchors for enhancement,” adding that even if it comes with a moderately increased risk of type 1a endoleak, it “balances against downsides of F-EVAR, CHIMPS, or against open repair (if F-EVAR is not an option).”
Discussing the outcome of the debate and the audience’s strong position against the motion, Blankensteijn said, “I think that people understand that things are not absolute and clearly we know that staying inside IFU you get better results. I am not running around proposing to treat any less than 10mm neck with an infrarenal device, but the point is that judicious use of these devices outside IFU can produce better results and injudicious use outside the IFU is an instruction that the industry gives you, we need to think with these device what to do an there are several occasions it does not make sense or it may even be dangerous to use a fenestrated stent graft. Then I would suppose that an infrarenal device (for instance Gore Excluder) preserves all the options juxtarenally, so if this fails, you still have the option to use a fenestrated cuff or go higher up.”
The debate took place during a session on procedures for infrarenal abdominal aortic neck.
The answer lies in the neck
While presenting the morphology findings from the IMPROVE trial, Robert Hinchliffe, London, UK, drew attention to independent association between neck length and mortality. “Only aortic neck length is significantly associated (inversely) with 30-day mortality both for open repair (p<0.001) and overall (p=0.007). The shorter the neck, the higher the mortality after open repair (and EVAR). With long necks, the 30-day mortality from EVAR and open repair is similar (and this is consistent with the results of the AJAX and ECAR trials),” he said.
Therefore, added Hinchliffe, a short aneurysm neck was the commonest reason for a patient being unsuitable for conventional EVAR. The results also explain why observational studies, which cherry pick long-necked aneurysms for EVAR leaving all the short necked aneurysms for open repair, always show that mortality is lower after EVAR. “Such observational studies are comparing apples and oranges,” he said.
The morphology findings could also explain the worse outcomes in women as short aneurysm necks are especially common in women.
“In the future, new, widely available endovascular strategies for rupture in short necked aneurysms are needed and results following rupture should report juxta-renal and infra-renal aneurysms separately,” Hinchliffe said.
“Aneurysm morphology indicates whether a patient with ruptured abdominal aortic aneurysm is eligible for EVAR and may influence the outcome of both EVAR and open surgical repair,” he concluded.
Stick to the IFU
Timothy Resch, Malmö, Sweden, enforced the point that the outcome of EVAR is “excellent if we stick to the IFU and hostile sealing zone anatomy affects the outcome of EVAR negatively both in the short and long term regardless of the device that you use.”
Resch explained that 10—20% of EVAR patients have necks <15mm, and these short infrarenal necks are predictive of initial technical failure, with increased incidence of early and late type 1 endoleak and increased use of intraoperative adjunctive procedures, where the long-term outcome is impaired and endoleaks, migration and late rupture are common.
Referring to a contemporary meta-analysis of 12,000 patients treated with modern devices, 3,039 patients with hostile necks saw an increase in 30-day mortality, intraoperative adjuncts, 30-day migration and an increase in type 1 endoleak at 30 days and one year.
Further, in the ANCHOR registry, Resch noted, a regression and ROC analysis was used to try to find predictive factors for type 1a endoleak which showed that the diameter of the neck correlates with poor outcome, as does the anatomic neck length. “This actually provides some solid data for calculating the risk in the individual patient,” he said.
“So one option of course in these short juxta pararenal aneurysms is to seal higher above and use the fenestrated and incorporate the visceral arteries in your repair. Does that solve the problem of the proximal endoleaks? Again, it depends on how you use the graft,” Resch suggested.
Making a case from some of his own data and that of a study at the Cleveland Clinic, USA. “We found no cases with proximal type 1 endoleaks during long-term follow-up of these patients. So maybe this is the solution for everything—using it in the right context. In a larger series from the Cleveland Clinic analysing over 900 patients with fenestrated grafts, indeed there was a 2.8% incidence of late type 1a endoleak occurring in a steady fashion over time and almost half after a year post-operatively. Looking at the risk factor in that series, what they identified again was a poor sealing zone, just as you would in an infrarenal neck and more that 10% was diameter change and they also found that the sealing zone was much more unstable in the juxtarenal vs. more proximal aorta. So from the clinical perspective, we have learned from that and we now place more fenestrations on the graft to reach a healthy sealing zone, which in our series, combined with the series from Lille with 300 patients, resulted in a higher placement of the endograft than we initially did without affecting the operative mortality or the one-year outcome,” he reported.
Three-year results of the INNOVATION study confirm earlier “very promising” findings
cxsymposium2015-07-11T02:23:23+01:00The INNOVATION study found that the rate of freedom proximal type I endoleaks and the rate of freedom from reintervention in patients who underwent endovascular aortic aneurysm repair (EVAR) with the Incraft (Cordis) device was, respectively, 100% and 95%, at three years. In this interview, CX Daily News speaks to Giovanni Pratesi (Florence, Italy)—who presented the data at CX yesterday—about Incraft and the three-year results.
How does the Incraft stent graft differ from other EVAR devices?
It is a new-generation stent graft system with unique features that have been specifically designed to overcome the limitations of current stent grafts. For example, the ultra-low profile integrated delivery system (14F OD) offers excellent navigational opportunities in challenging access vessels. Additionally, the micrometric deployment system allows for accurate placement—either at proximal and distal attachment site. Bilateral in-situ length adjustment, up to 3cm, permits you to customise every implant on the single patient anatomy. Therefore, the Incraft system, according to the “few-fits-most” concept, is able to cover a large spectrum of anatomies with only 23 product codes, four main bodies and 19 iliac limbs.
What are the main results of the INNOVATION study at three years?
The three-year results of the INNOVATION study have confirmed the very promising one- and two-year outcomes of the study. These data show, at three years, the device is associated with 100% freedom from type Ia and III endoleaks, stent-graft migrations, and device- or procedure-related major adverse events. They also show that the rate of limb patency is 97.8%, with only one case of limb occlusion. Other findings from the three-year data indicate that there is a significant reduction in mean aortic aneurysm diameter, up to 15 mm, compared with two-year data, a freedom from sac increase of 95.6% and freedom from stent fractures of 97.7%. Core lab analysis has identified two cases of aneurysm sac enlargement—both associated with a persistent type II endoleak—and one case of stent fracture, but these events did not appear to have any clinical consequences.
What other data were included in your presentation?
I specifically addressed endograft durability and anatomical preservation in my analysis of the Innovation study. Data from core lab analysis have confirmed the excellent stent graft stability that has already been observed during follow-up. A median proximal migration of 2mm was observed at three-year compared with the one-month computed tomography (CT) scan. The same result was achieved in terms of distal migration with a median change of 1.1mm and 2mm on the right and left side respectively, compared with the one-month CT scan.
Aortic neck diameter, neck angulation and iliac artery diameter, compared with one-month CT scan, were analysed at three years to review endograft influence on anatomic changes during follow-up. The observed 1mm proximal neck dilatation and 2mm iliac artery dilatation, combined with the 0.6 degree changes for infrarenal neck angulation and 2.4 degree for suprarenal neck angulation, confirmed the excellent stent graft conformability to the preoperative anatomy.
What were the main take-home messages from your presentation?
These data confirm the very promising earlier outcomes of Incraft stent graft system and add new evidence regarding its effectiveness in terms of anatomy preservation with a 100% freedom from both proximal aortic neck and iliac dilatation, and from proximal supra- and infrarenal aortic neck angulation changes.
CX Venous Workshop draws in the crowds
cxsymposium2016-10-12T13:01:16+01:00Yesterday saw the beginning of the two-day CX Venous Workshop, in which delegates can enjoy expert demonstrations of a selection of the most interesting and important phlebological technologies currently in use. Now in its seventh year, the event continues to attract more and more delegates to its plethora of new and established technologies, and this year occupied its largest space ever on the Upper Level of the Gallery. The first day of the workshop largely focused on varicose vein treatment and superficial venous issues.
Ian Franklin (Imperial College, London, UK) is leading the workshop over both days, and has done since it first featured at the Charing Cross Symposium seven years ago. He explained, “I think it is important to provide a combination of the big plenary sessions in the hall and smaller interactive workshops like this one—not all techniques and discussions lend themselves the plenary environment. By providing these training stations and this small-group one-to-one teaching format, it means that people can interact in a much more informal, personal level.”
Over its seven years the workshop has grown and changed, keeping pace with the changing face of the vascular field. “The needs have changed,” Franklin commented. “When we first started doing this it was an office-based varicose vein course. Now it is far more than that. We have found that quite a lot of the techniques that people were hungry to learn at the beginning are ‘old hat’ now, so each year we offer new things. Now people are hungry to learn deep vein stenting, intravascular ultrasound, non-thermal techniques for treating veins, and so on. This year we have some completely new things that people will not have seen before.”
Given the quality of the Charing Cross faculty, such one-to-one interaction presents an exciting educational opportunity. “All of our faculty are well-known names and enthusiasts in their field. You can interact with them much better if you talk to them face-to-face rather than if they are up on a podium and you are down in the audience and asking the questions through a microphone. This way you can ask things you might not be able to go into in a lecture theatre.” Franklin added that these face-to-face interactions combine with practical experience, and delegates can “hold the kit in their hands, practice on the simulator and talk through tips and tricks with someone who has done it a lot before—you cannot do that in a lecture theatre.”
The workshop’s layout with multiple training stations also means that delegates can be efficient with their time, heading straight for those stations dealing with subjects they are unfamiliar with. “They do not have to sit through things they already know about, so they can make very efficient use of their time,” Franklin said.
The workshop features both established and new technology. One of the busiest stations yesterday was that of a well-established technology—foam sclerotherapy. Simon Ashley (Plymouth, UK), was one of the trainers at the station. He spoke to CX Daily News during the workshop, and explained, “Personally it is the first time that I have been involved as a trainer, but I know that it has been running for a number of years and has grown increasingly popular—that is why so much space has been devoted to it.” As to why the station was so busy, Ashley noted that foam sclerotherapy “is a walk-in walk-out treatment that only takes about 20 minutes and there is no down time or restriction of activities afterwards. It is also very attractive for NHS and private practice because it is undoubtedly the most cost-effective treatment.”
In keeping with the Charing Cross Symposium’s passion for innovation, there was also a selection of new techniques and products on show. The Biolas station, exhibiting the company’s non-thermal ablation VariClose vein sealing system, was kept busy throughout the day by curious delegates. The company are attending their first ever Charing Cross Symposium, and chief medical officer, Yunus Çakiroĝlu, told CX Daily News “We are new this year, so I think a lot of people are wondering about our products and our company—they want to find out who we are and what we have at our station. The meeting is very exciting, and the workshop is a great way for the delegates to understand new technology.”
The workshop will continue today with a focus on acute deep vein thrombosis interventions, intravenous ultrasound and deep venous stenting, but still including ablation technologies for superficial veins. The stations open for delegates to visit will include: thermal (laser), thermal (radiofrequency ablation), non-thermal, practical training stations, valves, vascular ultrasound training simulator, venous malformations, pelvic venous imaging, pelvic vein embolisation, acute deep vein thrombosis, caval filters, follow-up protocols, air plethysmography, intravascular ultrasound and deep vein stenting.
Franklin expects the Upper Gallery to be just as busy as it was yesterday thanks to the planning of this year’s programme. He said, “We have made an effort to separate the days this year, with Wednesday focusing more on superficial techniques. On Thursday we are going to have a big focus on deep vein stenting, deep vein thrombosis treatments, pelvic vein embolisation and intravascular ultrasound. These are all quite new for us and should attract a new group, so I expect it to be busy tomorrow as well.”
Still few useful indicators of how to prevent aneurysm growth and rupture
cxsymposium2016-10-12T13:01:16+01:00According to Janet Powell, Imperial College London, UK, the highest risk factors for rupture of abdominal aortic aneurysms (AAA) are female gender, smoking, increasing age and mean arterial pressure, but there are still few useful indicators of how to manage screen-detected aneurysms to stop them enlarging. Powell put forward this view during yesterday’s Abdominal Aortic Controversies Main Programme which explored epidemiology, indications and medical management of abdominal aortic aneurysms.
In trying to work out what makes aneurysms grow, Powell said that mostly the “mundane” cardiovascular risk factors have been looked at—alcohol, diet, obesity, smoking, medicines taken, exercise taken.
Turning to the existing and ongoing randomised trials that provide evidence for growth factors, Powell pointed out three trials already completed and without a positive result—AAA:STOP, PHAST and AORTA.
“Even in 2015, it is quite difficult to find information about trials because some trials still remain unregistered. Large epidemiological studies have mainly shown us about the risks for developing aneurysms, but the focus has been on baseline risk factors, not aneurysm progression,” she said.
Powell referred to RESCAN, a large international project primarily with the purpose of looking at optimal surveillance intervals for small aortic aneurysms, which was an individual patient data meta-analysis of small aneurysms, their growth and rupture in more than 15,000 persons.
“As we all know, growth rate increases with aortic diameter—just 1.34mm a year for the smallest aneurysms and 3.63mm when it comes to 5cm aneurysms—with exactly the same growth rate between men and women,” she pointed out.
When it comes to smoking, Powell maintained that the data are remarkably consistent that it increases aneurysm growth rate but that the effect is modest. Similarly, the effect of diabetes to slow or reduce the aneurysm growth rate is very consistent, but small.
“So we identified out of the available baseline data we had only two factors in these 15,000 patients that influenced growth rate and both effects were relatively modest—smoking increases, diabetes decreases. Cholesterol, blood pressure, statins, anti-hypertensive drugs and aspirin had no effect, and the year of enrolment [1985–2008] had absolutely no effect either. Disappointing,” Powell stated.
Rupture rates
Even though rupture of small aneurysms is not common, findings were more informative. RESCAN showed that the smallest of aneurysms take eight or more years before the risk of rupture is anything substantive, but as the diameter increases, so does the risk of rupture. But even for a 5cm aneurysm, it is more than year before risk of rupture exceeds 1%.
Powell pointed to some strong associations with factors that increase rupture rates. “Most notable, the fact that in women, there is fourfold increase in rupture rates vs. men. With smokers there is a twofold increase, with a far bigger effect on rupture than on growth. Mean arterial pressure increases growth rate, as does age. The older you are, the more likely your aneurysm is to rupture for any given diameter. So we cannot have any of this ageism, we need to treat aneurysms in older people too,” Powell maintained.
In the EVAR 2 trial, and subsequently reproduced in other papers, Powell observed that aortic neck length was associated with rupture and the shape of the aneurysm might have mattered, and a long neck was associated with relative protection from aneurysm rupture.
“Where are we in 2015? For rupture, there are some strong factors increasing risk: female gender, smoking (which we can do something about), increasing age, higher mean arterial pressures (we could have more effective blood pressure control), and the fact that a long aneurysm neck could just be protective. However, for growth, the effects that we have identified are modest: smoking, which increases it, and diabetes, which is protective,” Powell said.
She re-stressed that both of those growth effects are modest, adding that, “unfortunately this leaves us in a position that as yet we have few useful indicators of how to manage screen-detected aneurysms to stop them enlarging other than the old classic that was used for intermittent claudication—stop smoking and possibly keep walking.”
One-year IMPROVE data suggest benefit of EVAR in ruptured aneurysms
cxsymposium2015-07-11T02:23:23+01:00Results from the 12-month data of the randomised controlled IMPROVE trial, presented for the first time at CX 2015 (28 April–1May, London, UK), show that an endovascular strategy is cost-effective when compared to open repair in the treatment of ruptured abdominal aortic aneurysms. The one-year data also revealed that the endovascular strategy conferred no survival benefit over open repair, except a trend towards benefitting women. It also showed that the endovascular strategy enabled more patients to be discharged from hospital to home, and significantly faster than with open repair. Patients also have an excellent quality of life with endovascular strategy, if they survive the rupture, delegates heard.
Twelve-month outcomes from the individual patient data meta-analysis of three randomised controlled trials of ruptured aneurysms [ie the Dutch AJAX trial, the French ECAR trial and the UK IMPROVE trial] also showed a trend towards survival benefit for endovascular aneurysm repair (EVAR), but this was not statistically significant. A CX audience poll in the session revealed that nearly 80% agreed that the 12-month results of the IMPROVE trial encouraged them to perform EVAR more often.
Janet Powell, Imperial College, London, UK, presented the latest data from the IMPROVE (Immediate management of the patient with rupture: Open vs. endovascular repair) trial that reported one-year outcomes following either a strategy of endovascular repair first or open repair of ruptured abdominal aortic aneurysm. The results demonstrate no survival benefit for the endovascular strategy for ruptured aneurysm at one year. However, an endovascular-first strategy for the management of ruptured aneurysms does offer patients faster discharge with better quality of life and it is also cost-effective. Both these factors are necessary for patient and clinical decision-making, delegates heard. These data were published in April in the European Heart Journal (EHJ).
IMPROVE, a pragmatic, multicentre (29 UK and one Canada) trial randomised 613 patients with a clinical diagnosis of ruptured aneurysm, 316 patients to an endovascular-first strategy (if aortic morphology was suitable and open repair if not) and 297 to open repair. Powell also drew attention to the fact that at baseline, the characteristics of the two groups were similar with almost three-quarters in each group being men. “The aneurysms in this trial were also very large with average diameters in each group being over 8cm,” she said. The principal one-year outcome was mortality and secondary outcomes were reinterventions, hospital discharge, health-related quality-of-life, costs, quality-adjusted-life-years and cost-effectiveness.
At CX 2015, Powell noted that the trial was designed to answer the question of what to do with a patient who presents to the emergency room with a diagnosis of ruptured abdominal aortic aneurysm. “What we had anticipated was that using an endovascular strategy, wherever it was morphologically feasible, we could reduce 30-day operative mortality from 47% in the open repair group to 33% where endovascular repair was used extensively,” she said.
Powell added: “At one year, all-cause mortality was 41.1% for the endovascular strategy group and 45.1% for the open repair group (p=0.325) with similar reintervention rates in each. A subgroup analysis showed a stronger benefit for the endovascular strategy in women versus men. The endovascular strategy group and open repair groups had average total hospital stays of 17 and 26 days, respectively.
The 30-day mortality results, the primary outcome, previously published in British Medical Journal (BMJ) in 2014, also found no difference in 30-day mortality between the endovascular strategy group and the open repair group and subgroup analysis showed a strong benefit for the endovascular strategy in women compared with men.
Pooled individual patient data outcomes
Ron Balm presented the 12-month outcomes meta-analysis from the individual patient data from the three randomised controlled trials of ruptured aneurysms; the Dutch AJAX trial (Annals of Surgery 2013), the French ECAR trial (EJVES 2015) and the UK IMPROVE trial.
“These were all trials that were performed on patients with a clinical suspicion of ruptured aneurysm. In the AJAX trial the 30-day mortality in both groups was approximately 25%, in ECAR it was 22% and in IMPROVE, which had a slightly different study design, it was 35% in each group (BMJ 2014), but all failed to demonstrate the benefit of EVAR in the emergent setting. “The results were hampered by the fact that open repair performed so much better than we expected,” Balm noted.
The pooled inpatient data was subjected to two different analyses; the first was an analysis as randomised including all patients and the second contained only those patients with proven rupture and a restricted cohort from the IMPROVE trial who were anatomically suitable for EVAR.
“The 30-day survival data showed no significant benefit for endovascular repair. A subgroup analysis of age, sex and Hardman disability index showed that age and Hardman index had no influence on the main results, but female sex was identified as benefitting from endovascular repair. The time to discharge alive from primary admission was also significantly better for patients undergoing EVAR when compared to those undergoing open repair. We also looked at the influence of neck diameter, neck length and neck angulation. Longer neck lengths resulted in better survival for all patients,” Balm said.
In the pooled results of all patients, the three-month mortality rate included only those patients who were fit for endovascular repair, which reduces the patients in the IMPROVE trial. There seems a benefit for endovascular repair but this is statistically not significant, he said.
Summarising the survival results of all pooled patients to one year, Balm said, there is an advantage for EVAR, but this is statistically not significant.
These findings led Balm to conclude that the early data from the pooled inpatient data meta-analysis did not suggest that there was any survival benefit for EVAR. “At one year, the small survival advantage of EVAR is not significant. Yet, although endovascular repair does not offer a significant survival advantage, endovascular repair should be used more widely. Open repair must remain available for those unsuitable for conventional EVAR,” Balm maintained.
Are patients with ruptured aneurysms being turned down too often?
A small majority of voters, 57%, did not agree with the motion “Too many patients with ruptured aneuryms are denied intervention” in an interesting debate on the topic.
Matt Thompson, London, UK, speaking for the motion, based his arguments on the data from Karthikesalingam et al (The Lancet, 2014), which used UK Hospital Episode Statistics (HES) data and US Nationwide Inpatient Sample (NIS) data to show that in-hospital survival from ruptured abdominal aortic aneurysm, intervention rates, and uptake of endovascular repair are lower in England than in the USA.
Thompson stated that 68% of patients in the UK receive no treatment for rupture. “There is a huge variation in the proportion of patients being offered therapy for ruptured abdominal aortic aneurysm at the regional and international level. Non-interventional treatment is universally fatal,” he said.
Thompson argued that apart from identifying the optimal management strategy for patients with ruptured aneurysms, more emphasis should be placed on treatment rates. “We must do better than refuse treatment to around 60% patients,” he said.
Peter Lamont, Bristol, UK, who persuaded the delegates that ruptured aneurysm patients were not being denied interventional treatment too often, contested the data presented by Thompson because with the HES data it made the assumption that the diagnostic code for ruptured abdominal aortic aneurysm without an operative code for the condition was a turndown. He also pointed out that a 2005 coding audit had found up to 23% HES codes to be incorrect. Further, NIS data misses 80% of patients, noted Lamont.
“Numerous direct audits in 2010 and after in the UK show consistent turndown rates of around 20% by vascular surgeons,” he said.
Lamont pointed to a Vascular Society members audit in 2011 that revealed a turndown rate of 24% and also showed that the average age of patients was 83. “Of these, 24% declined intervention, 14% had dementia and 21% had terminal malignancy/severe chronic disease,” he added.
Lamont concluded by saying that the service reconfiguration in the UK guarantees that vascular surgeons are now on call 24/7 for ruptured abdominal aortic aneurysm. “When comparing like for like, the turndown rates in the UK are similar to those in the USA,” Lamont said.
In the ensuing discussion, Powell made the point that twice as many women than men are turned down for treatment when they present with ruptured aneurysm and asked for a response on why this might be the case. “We did not formally address that in the IMPROVE trial, but there are several observational cohorts to suggest that adopting a more minimally invasive approach would lead to a reduction in turndown rates in these patients,” added Thompson.
Thompson explained the criteria for palliation; patients in whom intervention is completely futile, such as those with advanced malignancy or dementia, who have no quality of life are suitable candidates for palliation. “There are patients in whom it is cruel to intervene as they do not want their life prolonged,” agreed Lamont.
Frank Veith, New York, USA, made the point that the way to decrease the turn down rate for ruptured aneurysms was to embrace endovascular repair.
Bioresorbable scaffolds are “the only complete solution for the superficial femoral artery”
cxsymposium2016-10-12T13:01:16+01:00The use of bioresorbable scaffolds is the only complete solution for the superficial femoral artery, argued Andrew Holden, Auckland, New Zealand, in yesterday’s Peripheral Arterial Controversies session.
Presenting an update on the Stanza programme, Holden told delegates that the ideal treatment strategy for superficial femoral artery disease has been the source of much research, discussion and controversy. “An endovascular device should provide vessel support acutely to manage dissection and recoil, include an anti-restenosis strategy and preferably leave nothing behind when no longer needed. A bioresorbable drug-eluting scaffold potentially fulfils these requirements,” he explained.
The programme employed the Stanza platform (480 Biomedical)—a flexible, self-expanding stent design with full resorption in about 12 months. Holden summarised the development of the Stanza scaffold, the first part of which—the STANCE first-in-man trial—allowed “assessment of stent parameters such as precise positioning and deployment, excellent radial resistive force with minimal residual stenosis and satisfactory resorption.”
Two sub-studies in the STANCE trial allowed original validation of important imaging modalities by independent core laboratories. One sub-study analysed the accuracy of quantitative vessel analysis of magnetic resonance angiography (MRA) compared to the gold standard catheter angiography. A second sub-study compared cross sectional luminal area evaluation using MRA and optical coherence tomography (OCT). This comprehensive analysis, Holden explained, confirms MRA is “an effective method to assess the vessel lumen non-invasively after treatment with a bioresorbable scaffold.”
The study showed 100% scaffold delivery success, good scaffold apposition verified by optical coherence tomography (OCT) and angiography, and acute performance similar to metal stents. Holden noted that OCT is a vital tool in the assessment of bioresorbable scaffolds, as it allows investigators to identify scaffold encapsulation during healing and resorption. Using OCT Holden was also able to create 3D reconstructions of the vessel, allowing him to detect any scaffold fractures and show that at six months the scaffold demonstrated chronic strength to prevent vessel recoil.
The drug-eluting version of the Stanza platform is currently being assessed in the SPRINT clinical trial. Holden told attendees that the biggest challenge has been to define appropriate and extended drug release kinetics to deal with the inflammation associated with scaffold resorption. This has been achieved with the drug eluting version of Stanza. Currently, there is ongoing recruitment and evaluation in this trial, as well as several examples with medium-term follow-up.
In one such case study, pre-implant the patient had 88% stenosis, a figure which fell to just 2% residual stenosis following the implant. Similarly, in the second case that Holden presented, the pre-implant stenosis figure was 92%, falling to 0% following the implantation of Stanza. Both of these case studies were evaluated with MRA and OCT to confirm the outcomes.
The best of the new: CX Innovation Showcase features the latest technologies
cxsymposium2019-11-01T11:11:00+00:00The CX Innovation Showcase is dedicated to highlighting what it takes for a physician-inventor to succeed and for innovative ideas in the vascular and endovascular arena to thrive. Yesterday’s session brought together leading industry experts, physician-inventors, engineers, medtech investors and start-up companies all focused on improving vascular disease management.
Chaired by Stephen Greenhalgh, the 2015 Innovation Showcase covered innovations across five areas: endovascular aortic, complex aortic endografting, peripheral, diagnostic and vascular access.
Vascular access
Andrew Holden (Auckland, New Zealand) presented for the first time at a scientific meeting, the first-in-man experience with the Advance 35 scoring balloon (Cook Medical) in haemodialysis fistula stenosis. He noted that it is well known that stenosis in the haemodialysis access circuit is a common complication for patients on haemodialysis, adding that regular surveillance is important, especially after intervention because restenosis is common. Holden said that the para-anastomotic and outflow stenoses are often resistant to angioplasty using conventional angioplasty balloons at nominal pressures, and frequently, ancillary measures are required, such as scoring or high pressure balloon angioplasty. These ancillary procedures, he said, come at a financial cost as well as a morbidity cost related to upsizing sheaths and guidewire exchanges.
In the early experience with a 0.035” guidewire compatible scoring balloon, specifically designed for the management of fistula stenosis, 39 stenotic lesions in 28 patients were treated. Acute procedural success, as defined by a residual stenosis of <30% was achieved in 38 of 39 patients (97%).
“This compares very favourably to an audit of consecutive patients at the same centre treated with standard angioplasty balloons where procedural success was only 48%. In the trial only one patient required an ancillary procedure to optimise the result where 11 patients required cutting balloon or high pressure balloon angioplasty in the audit group,” Holden reported.
Optical coherence tomography in a sub-group of patients confirmed the scoring effect in treated vessels. At one month, patency was 100%. Six-month patency will be evaluated soon.
“These promising results suggest this 0.035” guidewire compatible scoring technology could become a ‘first line’ tool for the treatment of dialysis fistula stenosis,” Holden concluded.
Endovascular aortic
Bao Bui (Sherbrooke, Canada) made a case for in vivo antegrade fenestration of renal arteries in pararenal abdominal aortic aneurysms (AAA). He said that in vivo fenestration is associated with a low leak rate, it is easy to learn and to perform, there is no wait time using off-the-shelf devices, and the device is possible for emergency use.
He concluded stating that based on initial results (five patients, no leak and no migration 10 months follow-up, all AAAs and the TAAA have decreased in size), in vivo antegrade fenestration is a very promising, reliable technique that is easy to perform with existing devices.
Complex aortic endografting
Patrick Kelly (Sioux Falls, USA) looked at a paravisceral non-anatomical branch endograft system. Noting that repairing short neck, pararenal, paravisceral, and type IV thoracoabdominal aortic aneurysms is challenging because moving the seal zone more proximally increases the risk of paraplegia, he presented a novel approach for sealing just above the celiac.
“We envision the device to be completely off-the-shelf, to lessen or completely negate the need for rotational alignment, and deliver ordered, well-developed flow to the branch arteries. Until the devices are pre-manufactured, we are using physician-modified versions for patients with limited options. The device is made with a diaphragm, which allows for minimal aortic coverage,” Kelly said.
He explained that the paravisceral stent graft configuration is built from a 28–36mm Endurant stent graft (Medtronic) which is shortened to 30mm with the suprarenal fixation hooks left attached. The diaphragm is created from an ePTFE vascular patch. The lumens are made from Viabahn stent grafts (Gore). The paravisceral stent graft is introduced through the groin. The bridging stents for the renal arteries, SMA, and coeliac are introduced through the arm. Once the visceral segment is excluded, a standard EVAR is performed to exclude the remainder of the infrarenal aorta.
“Our experience to date has been one of excellent technical success and early branch vessel patency,” Kelly concluded.
Pierre Galvagni Silveira gave the early clinical results of the TAMBE (Gore) thoracoabdominal branch endoprosthesis. He reported that at 30-day follow-up there was no endoleak and treated branch patency was 100%.
Silveira concluded stating, “The TAMBE device is the only ‘all-in-one’ pre-cannulated off-the-shelf system, including aortic component and side branch stent graft. While the initial results are promising, longer term outcomes are needed to confirm this early experience.”
Shin Ishimaru (Saitama, Japan) presented the Najuta endograft for total branch repair. He said that the primary concept of Najuta was to design a tailor-made endograft suitable for the aorta of each individual patient. “The proximal end of the device is placed between zone 0 and 2, which constitutes the highly critical region involved with the aortic arch and branch arteries. Fixation of the endograft is successful if there is at least 20mm of healthy aorta from the left carotid or subclavian arteries to the margin of the aneurysm,” Ishimura said.
He concluded adding that with almost 200 cases now done in Japan, and five-year data on the horizon, the Najuta fenestrated endograft is a “promising device for aortic arch application”.
Jan Heyligers (Tilburg, Netherlands) discussed spiral vein reconstruction using the greater saphenous vein to reconstruct a neo-aorta in infected cases. Heyligers noted that research has shown spinal vein reconstruction to be technically feasible and a successful alternative for the Glagett-Nevelsteen procedure. He said that the advantages are that no training is necessary, there is no learning curve, diameter is not an issue and there is no morbidity when harvesting the deep vein.
Peripheral
Stephen Williams (Baltimore, USA) discussed helical 3D stenting with the BioMimics 3D stent (Veryan), maintaining that the Mimics data indicate in-stent swirling flow/endothelial wall shear stress could be beneficial to maintain long-term patency. “This is really the only stent in 12–24-month range study to date that seems to offer this improvement in avoidance of late restonotic events,” Williams noted.
He later announced that the US IDE trial for the BioMimics stent is upcoming, with the first enrolment expected by the end of this quarter.
Patrick Kelly presented the early results of paclitaxel after endorevascularisation, concluding that drug infusion balloons could have great potential.
“We believe it to be more favourable [than drug-coated balloons] in many regards, including: shorter regulatory path, lower device cost, and more flexibility in the therapeutic used,” Kelly maintained.
He explained that he has been working on this project with two parallel paths: clinical research using an already available infusion balloon, and bench top and animal research to evaluate various excipients. “In the clinic, we have seen an improvement in freedom from binary restenosis and freedom from target lesion revascularisation. On the benchtop and in animals we have evaluated cremaphor, urea, contrast, and Abraxane.”
He reported that notably, “Abraxane shows superior tissue penetration in our animal models when quantified with a validated high performance liquid chromatography method. The Abraxane is also a superior in terms of biocompatibility without the need for any toxic solubility agents.”
The next step in the research will include a prospective randomised blinded clinical study of 150 limbs with de novo lesions.
Diagnostic
David King (London, UK) returned to the CX Innovation Showcase this year after his winning pitch in the 2014 Dragons’ Den. Yesterday, he presented his Blue Dop technology (with which he won the 2014 £1000 Dragons’ Den prize), and made a case for the technology’s empowerment of nurses in vascular investigations.
King said that Blue Dop empowers tissue viability nurses working in the community to start patient treatment immediately, thus reducing costs for GP practices, reducing the number of hospital referrals, speeding up the patient treatment “journey” and reducing the load on vascular labs.
Dragons slayed by new dialysis graft technology
In the tradition of the CX Innovation Showcase, the highly-anticipated Dragons’ Den did not disappoint in 2015 with presentations of 15 new and innovative ideas from physician-inventors from all over the world.
With much to consider after two hours of impressive presentations, Jeffrey Lawson (Durham, USA) came out on top, winning over the Dragons and gaining the most votes in his favour for his dialysis graft technology.
Lawson spoke to CX Daily News about his technology stating, “It is a novel dialysis graft that is meant to protect patients when their dialysis graft needs to be cannulated by a sharp needle. You can only cannulate the graft or the blood-flowing portion safely and reliably—that is the nature of the innovation.”
In terms of progress, Lawson reported that animal testing with the device has been done and a very mature prototype design has been developed. Further, he said that his team has met with the US Food and Drug Administration (FDA) in light of plans for the first-in-man implant.
Commenting on the value of the Dragons’ Den, Lawson said, “It is a wonderful opportunity, and it is fun. It is also really good to see other people’s technology making progress.”
Speaking to CX Daily News, course director and chairman, Stephen Greenhalgh said, “The feedback I received from the Dragons was that this year saw the most wide-ranging, innovative group of presentations that we have ever had at Charing Cross, and based on voting results—where varying presentations received recognition from the individual Dragons—it shows how closely fought it was.”
Speaking of the future of the CX Dragons’ Den, Greenhalgh added, “I just want to ensure that Charing Cross always provides a platform for early stage innovation.”
Honourable mention was also given to Peter Philips (Didcot, UK) for his ideal compression stocking; Tim Chuter (San Francisco, USA) for his Inchworm balloon; and Lindsay Machan (Vancouver, Canada) for his uniform pressure non-straightening angioplasty balloon.
DEFINITIVE AR suggests atherectomy before drug-coated balloons in calcified lesions confers benefit
cxsymposium2015-07-11T02:23:23+01:00Twelve-month results from the randomised, multicentre DEFINITIVE AR pilot study suggest that there is a benefit to adding directional atherectomy in long and calcified lesions prior to using a drug-coated balloon in comparison to the use of a drug-coated balloon alone. Data from the trial were presented yesterday by Thomas Zeller, Bad Krozingen, Germany, who is a study principal investigator alongside Gunnar Tepe, Rosenheim, Germany.
DEFINITIVE AR was designed to assess the effect of treating a lesion with directional atherectomy (Medtronic/Covidien’s SilverHawk or TurboHawk plaque excision systems) followed by a paclitaxel-coated balloon (Bayer HealthCare’s peripheral paclitaxel-coated angioplasty catheter with Paccocath Technology), collectively referred to as DAART (directional atherectomy + anti-restenotic therapy), in order to generate early hypotheses for further research in this therapy area.
Claudicants (Rutherford clinical category 2–4) with 7–15cm superficial femoral and/or popliteal lesions were randomised 1:1 to either DAART (n=48) or to the paclitaxel-coated balloon alone (n=54). Patients with severely calcified lesions were assigned to a non-randomised registry arm and were treated with DAART (n=19). Baseline patient and lesion characteristics did not differ between the DAART and drug-coated balloon arms.
Technical success, defined as ≤30% residual stenosis following the protocol-defined treatment at the target lesion, was significantly higher in the DAART arm vs. the drug-coated balloon arm (89.6% vs. 64.2%, p=0.004). According to Zeller, there were significantly fewer flow-limiting dissections reported in patients treated with DAART vs. drug-coated balloon alone (2% vs 19%, p=0.01). Technical success in the DAART registry group was 84.2% and incidence of flow-limiting dissections was 0%.
At 12 months, per cent stenosis in the randomised groups was 33.6±17.7 for the DAART arm vs. 36.4±17.6 for the drug-coated balloon arm. Duplex ultrasound patency (PSVR ≤2.4, without target lesion revascularisation) was 93.4% for the DAART arm and 89.6% for the drug-coated balloon arm. Angiographic patency (≤50% stenosis and without target lesion revascularisation) was 82.4% in the DAART arm and 71.8% in the drug-coated balloon arm. In the DAART cohort lesions with a residual stenosis <30% post atherectomy did show a trend towards better angiographic patency at one year (94.1% vs. 68.8%).
“This rigorously performed pilot study suggests an added benefit for DAART over drug-coated balloons alone in long and calcified lesions. Further investigation in larger, prospective, statistically-powered randomised trials is warranted. Patients will be followed out to 24 months to assess durability of the results,” Zeller said.
Cordis launches Outback Elite re-entry catheter at CX
cxsymposium2016-10-12T13:01:16+01:00At this year’s Charing Cross Symposium, Cordis is launching the Outback Elite re-entry catheter, an enhanced version of the Outback re-entry catheter that received the CE mark earlier this week. According to the company, Outback Elite provides more control and precision and includes additional features to enable re-entry into the most complex lesions whilst facilitating positioning and delivery.
Yesterday, Thomas Zeller, Bad Krozingen, Germany, a user of the Outback re-entry catheter, spoke to CX Daily News about his experience with the device and his expectations about this new iteration.
Amongst the new features of Outback Elite are single-handed torque and deployment—the torque control is located closer to the needle actuator and the longer handle provides a more efficient slider mechanism—a robust nitinol cannula for re-entry in very complex lesions and a lubricious hydrophilic coating to help delivery.
In addition, the Outback Elite is available in a 120cm shaft length as the previous catheter but is also being launched with an 80cm shaft length indicated for ipsilateral antegrade or iliac procedures. The new size offers the advantage of having less shaft length to handle outside of the patient, increasing precision on the re-entry site.
The Outback Elite follows the successful performance of the Outback catheter. In a randomised controlled trial by Roberto Gandini et al comparing re-entry with Outback vs. manual re-entry in chronic total occlusions in the superficial femoral artery, the device achieved 100% re-entry rate vs. 42.3% with the manual re-entry technique. It also showed shorter procedure and fluoroscopy times.
Kristen Rock, global product director at Cordis, told CX Daily News, “Outback Elite’s ergonomic handle and added shaft sizes provide physicians with more control, meaning they can easily pinpoint exactly where they want the re-entry site to be.”
The Outback re-entry device will be used in a live case today at the CX Live Peripheral Arterial Cases – SFA and popliteal in the Grey Learning Centre.
CX 2015 audience voting favours standalone use of drug-coated balloons only for short SFA lesions
cxsymposium2016-10-12T13:01:18+01:00Drug-coated balloon technology is unlikely to be a successful “stand alone” treatment for the majority of medium and long length superficial femoral artery lesions, the voting results from CX2015 implied. The voting showed that the audience felt that drug-coated balloon use was best suited to short lesions. The randomised controlled trial data for drug-coated balloons are mostly based on short lesions and 57% of the audience voted “no” to the question, “Is drug-eluting balloon technology likely to be a successful standalone treatment for the majority of medium and long length superficial femoral artery lesions?” The session in which the vote was held was titled “Leaving nothing behind” and also saw the presentation of the ILLUMENATE first-in-human study 24-month results. These were favourable for the Stellarex drug-coated balloon (Spectranetics), and came close on the heels of updates on the Lutonix drug-coated balloon (Bard) and the IN.PACT Admiral drug-coated balloon (Medtronic). Atherectomy as a pretreatment also received a boost.
For the first time, CX voters also found support for atherectomy, but as a pre-treatment to the use of drug-coated balloons as described in the DEFINITIVE AR trial or as a pre-treatment to the use of stents. The majority, 77%, supported the motion “pretreatment of superficial femoral artery lesions before drug-coated balloon has CX 2015 audience support.
Thomas Zeller, Bad Krozingen, Germany, explained that “Drug-coated balloons add benefit to the endovascular approach to treat femoropopliteal disease but that there are still limitations such as recoil, calcium and dissections.”
Previous research has shown that circumferential calcification is a negative predictor for successful outcome with drug-coated balloon angioplasty. “Directional atherectomy is one of the most interesting pretreatment plaque modulating or plaque removing options available, after cutting or scoring balloons have been shown not to be very effective at this. The removal of ex-centric plaque and calcified plaque serves to reduce the problem of early recoil that limits the use of drug-coated balloon angioplasty outcome,” he said. Zeller also noted that the large majority vote favouring the use of atherectomy as a pretreatment were akin to a mandate to the industry to support large-scale randomised controlled trial to prove this concept.
In previous years, atherectomy has failed to elicit CX voter backing as a definitive treatment option in the superficial femoral artery. In 2014, 57% of the CX audience voted “no” to the motion, “I would use atherectomy for some superficial femoral artery lesions”. In 2013, 68% voted against the motion “atherectomy is the answer for the superficial femoral artery technology”.
This year’s vote shows that there is increasing support for atherectomy as a pre-treatment option rather than a definitive treatment and that the technique is clearly currently in favour of being used prior to drug-coated balloon or stents in order to reduce the risk of thrombosis. A member of the audience asked whether atherectomy should be the first option for isolated popliteal lesions considering there was a desire to leave nothing behind. Zeller, responding, said that it was frequently observed that isolated popliteal lesions were focal calcified lesions and for that indication, he considered atherectomy “an excellent first option followed by angioplasty or stenting”. “However, if we are talking about evidence to support this, there is none,” he clarified.
CX delegates at the session also heard updates on the Stanza bioresorbable scaffold (480° Medical) and Shockwave Lithoplasty for calcified artery from Andrew Holden, Auckland, New Zealand.
ILLUMENATE
Stephan Duda, Berlin, Germany, presented the results of the 24-month ILLUMENATE first-in-human study. These showed that the primary patency rate (as measured by Duplex Core lab evaluation) was 80.3% at 24 months. At 12-months, the primary patency rate was 89.5%.
“The freedom from clinically-driven target lesion revascularisation rate (as determined by the clinical events committee adjudication) was 85.8% at 24 months and it was 90% at 12 months,” Duda said.
In the study, researchers evaluated Stellarex in 50 patients with 58 lesions in a cohort that required predilatation with an uncoated balloon before the inflation of the drug-coated balloon. The mean age of the cohort was 69 years. The mean age of patients was 69.0±9.3 years; Thirty four per cent of patients had diabetes and 80% had hypercholesterolemia; Eighty six per cent were Rutherford Class 3 at baseline; the mean lesion length was 7.2cm; nearly 14% had severe calcification and the mean stenosis at baseline was 75%.
The vessel patency was associated with a significant and sustained functional improvement as resulted by the walking impairment questionnaire and by the treadmill test patient subset. There were no cardiovascular deaths or amputations reported throughout the 24-month follow-up schedule in this cohort, demonstrating the high safety profile,” Duda said.
He continued: “This study demonstrates the safety and efficacy of Stellarex for the treatment of femoropopliteal disease up to two years. The primary patency of 89.5% and 80.3% match the highest benchmark of reported rates at one- and two-years respectively. There is also significant functional benefit observed with improved walking distance, observed up to two years.”
Duda told CX Daily News: “Stellarex employs a next-generation manufacturing technology and coating formulation. This combination allows for an effective dose of paclitaxel to be transferred to the treatment site using a low drug dose density of 2µg/mm2. Pre-clinical work has shown this coating results in high coating stability with limited drug loss and an effective amount of drug in the arterial wall through 28 days.”
There is a robust clinical programme for Stellarex that is actively underway and will include up to 1,300 patients in five studies, Duda concluded.
IN.PACT SFA clinical trial update
Gunnar Tepe, Rosenheim, Germany, presented the 12-month results from the pivotal randomised trial evaluating the safety and effectiveness of the IN.PACT Admiral drug-coated balloon.
“IN.PACT SFA is a rigorously designed trial that provides level I clinical evidence supporting the safety and effectiveness of the IN.PACT Admiral when used to treat lesions in the superficial femoral and proximal popliteal arteries,” Tepe said.
The trial is a prospective, randomised, international, 57-site trial comparing the outcomes of treating a lesion with the paclitaxel-coated IN.PACT Admiral vs. standard percutaneous transluminal angioplasty using an uncoated balloon.
A total of 331 patients with claudication or rest pain (Rutherford clinical category 2–4) with single de novo or non-stented restenotic lesions ≥4cm and ≤18cm or chronic total occlusions ≤10cm were randomised 2:1 to drug-coated balloon (n=220) or angioplasty (n=111). Data was independently evaluated by blinded duplex ultrasound and angiographic core labs and by a blinded clinical events committee.
Tepe said: “Baseline clinical, angiographic and lesion characteristics were well balanced between treatment groups. Mean lesion length was 8.94±4.89cm in the drug-coated balloon arm vs. 8.81±5.12cm in the angioplasty arm (p=0.815), representing the longest lesions evaluated to-date in a randomised drug-coated balloon trial.”
A total of 59.3% of lesions in the drug-coated balloon arm were calcified vs. 58.4% in the angioplasty arm (p=0.097), and 25.8% of lesions in the drug-coated balloon arm were total occlusions vs. 19.5% in the angioplasty arm (p=0.222).
“At 12 months, primary patency in the drug-coated balloon arm (freedom from clinically-driven target lesion revascularisation and freedom from restenosis as determined by duplex ultrasound PSVR≤2.4) was superior to the angioplasty arm (89.8% for the drug-coated balloon vs. 66.8% for the angioplasty arm, Kaplan-Meier analysis at day 360 showed that p<0.001). Clinically-driven target lesion revascularisation was statistically lower for the drug-coated balloon arm vs. the angioplasty arm (2.4% vs. 20.6%, p<0.001). Regarding clinical endpoints, we can conclude that patients treated with IN.PACT Admiral perform more favourably than angioplasty in terms of quality of life and walking capacity and angioplasty treated patients require 88% more interventions to achieve the same clinical outcome,” Tepe said.
“Patients are being followed out to five years post-procedure and two-year data are forthcoming,” Tepe concluded.
Lutonix update
Dierk Scheinert, Leipzig, Germany, spoke on the topic “New insights from Levant 2 randomised data” that included data from subgroup and post-hoc analysis. “Levant 2 demonstrated superior patency for Lutonix against angioplasty with a 29.4% improved patency over angioplasty. The device was effective in a challenging patient population and showed target lesion revascularisation rates of 89% that were consistent in both the clinical trial and real-world registry,” he said.
Scheinert noted that Levant 2 had demonstrated the safety of Lutonix showing low rates of re-intervention for thrombosis and embolism. “Patients who received Lutonix DCB reported clinical benefits in Levant 2 and showed sustained improvement in Rutherford class and improvement in self-reported walking distance scores” he said.
Fabrizio Fanelli, Rome, Italy, outlined the current status of drug-coated balloon use in the superficial femoral artery. “There are more than 10 drug-coated balloons that are commercially available in Europe. All of them are based on the use of the same drug, paclitaxel, but the dosage is different, varying between 2µg/mm2 and 3.5µg/mm2. Moreover, each drug-coated balloon has a different excipient and a different coating technology,” he said. Most drug-coated balloons have passed their proof-of-concept test, but few have offered high-quality clinical evidence and programmes, even fewer have delivered best-in-class outcome, he emphasised.
Fanelli highlighted that studies such as IN.PACT SFA, LEVANT-2 and ILLUMENATE FIH were all multicentre studies with results that have been validated by independent core labs and that these data were essential in evaluating the quality of evidence and the quality of outcomes. “Only the most reliable data must be taken into consideration. There is no drug-coated balloon ‘class effect’. Each drug-coated balloon is to be judged upon the existence and value of its own data,” he stated.
Drug-coated balloons are cost-effective
Michael Jaff, Boston, USA, told CX 2015 delegates that while there are no prospective data collection studies, two publications have demonstrated that drug-coated balloons are a cost-effective alternative to angioplasty.
Jaff said: “Using current modelling, drug-coated balloons cost the system less than angioplasty, bare metal stents and drug-eluting stents, due to the lower target lesion revascularisation rates. It is critical that future randomised trials of one revascularisation technology include direct measurements of cost of care.”
He told delegates that the future was to be all about economic value and that in the previous week, the US payer system was in the process of reviewing the cost, efficacy and outcomes of all peripheral artery interventions for patients with intermittent claudication.
Jan B Pietzch and colleagues, publishing in Catheterization and Cardiovascular Interventions (2014), in studying the impact on payers and providers of the four main endovascular strategies for the treatment of infrainguinal peripheral artery disease in the USA and Germany, showed that drug-coated balloons and drug-eluting stents, compared to bare metal stents and percutaneous transluminal angioplasty, are associated with lower probabilities of target lesion revascularisation and therefore, cost savings for payers. Another publication, from BC Kearns and colleagues, published in the British Journal of Surgery in 2013, that set out to perform an economic evaluation of the cost-effectiveness of endovascular enhancements to percutaneous transluminal balloon angioplasty with bail-out bare metal stents for infrainguinal peripheral arterial disease, found that the use of drug-coated balloons represents a cost-effective alternative to the use of angioplasty with bail-out bare metal stents.
Jaff made the point that comparing the cost of using drug-coated balloons with the costs of percutaneous transluminal angioplasty, bare metal stents and drug-eluting stents was a “complex proposition”.
“Device costs are only one part of the equation. If the procedure offers greater durability, as defined by reduction in clinically driven target lesion revascularisation, the cost of care for that patient with peripheral artery disease will be lower than a comparable device requiring repeat interventions over a defined period of time,” Jaff concluded.
Direct balloon angioplasty
The panel agreed that more data were needed on whether pre-dilatation was needed before drug-coated balloon angioplasty was performed or not and called for a trial to compare the outcomes achieved with drug-coated balloon use after pre-dilatation to those achieved with direct angioplasty with drug-coated balloon.
Leaving something in
Speaking on the paradigms and importance of lesion lengths and types in the afternoon session on increasing lesion length options and various stent controversies, William Gray, New York, USA, made the point that lesion length is the only clearly identified driver of long-term outcomes. He drew attention to the fact that “Short-term outcomes may also be affected with increasing stent usage with increasing lesion length. Chronic total occlusion and diabetes do not appear to effect outcomes. The calcification grading is problematic, but likely has implications for both short- and long-term outcomes and adjunctive/specialised therapies may mitigate this effect.
Holden noted that five years ago lesion length had had a direct impact on patency with the technology that was available then. “Now with an armamentarium that consists of drug-coated balloons, biomimetic stents, drug-eluting stents, covered stents and atherectomy, we are saying that that paradigm is changing. Are we seeing less impact of lesion length on patency?” he asked. Gray noted that in the main, lesion length was the main driver of patency but that newer technologies and adjunctive therapies could modify the longer lesions in the drug-coated era and that he remained optimistic about this development.
Edited live aortic and carotid cases presented at CX for the first time
cxsymposium2016-10-12T13:01:18+01:00CX’s new Edited Live Case session, which aims to highlight the technical aspects of the aortic and carotid main programme, was launched yesterday with a selection of cases exploring how to apply different techniques in complex situations to achieve the optimum result for the patient.
Roadsaver – Carotid stenting with micromesh
The Roadsaver (Terumo), a self-expanding stent that has a dual layer micromesh design for sustained embolic protection, was used to treat a 73-year-old female with a high-grade symptomatic lesion in the internal carotid artery, Arne Schwindt (Munster, Germany) reported. He explained that she had an ipsilateral Amaurosis fugax eight weeks’ prior to the intervention and computed tomography (CT) angiography and Duplex confirmed a 80% right internal carotid artery stenosis. The patient also had arterial hypertension, hypercholesterolaemia, and type II diabetes.
Further to a having dual mesh design, Schwindt commented, the Roadsaver has “a flexible weave completely made of nitinol, an extremely flexible delivery system, and is retrievable and repositionable”. He explained that although accessing the lesion was the key challenge in the case, the risk of dislodging the stent’s guidewire system was low because of the system’s flexible delivery system.
According to the six-month follow-up result, the patient had no neurological events during the peri or post procedural period. Furthermore, convergent colour Doppler showed that the stent was patent with vmax of <100cm/seconds (previously, it was 190cm/sec). Schwindt concluded that the stent has “proven good deliverability, wall adaptation, and can be used with a variety of protection devices in challenging anatomies and lesions.”
Relay – Scalloped thoracic graft to the left subclavian artery and branched arch procedure
Mo Hamady and Joost van Herwaarden (London, UK, and Utretch, The Netherlands, respectively) jointly presented the case of a 43-year-old female patient with known hypertension who arrived at the hospital after she fell from steps at home. Because the patient presented with back pain, CT angiography was performed and this showed a non-traumatic saccular aneurysm of the thoracic aorta (ie. ductus diverticulum).
The presenters explained that the 9.5cm type 1 thoracic aortic aneurysm involved type III aortic arch, starting from the level of the innominate artery and extending to the distal thoracic aorta. There were more than 90 degree double bends at the distal end of the aneurysm.
According to van Herwaarden, the distance from the left subclavian artery to the aneurysm was too short for thoracic endovascular aortic repair (TEVAR) below the subclavian artery, and “further the ‘young’, highly curved aortic arch might have caused bird-beaking of a stent graft deployed in the aortic arch”.
Treatment options were TEVAR with covering of the left subclavian artery with or without revascularisation, TEVAR using a custom-made stent graft with a scallop for the left subclavian artery, or open repair.
The Relay (Bolton Medical) device, a custom-made branched thoracic stent graft with proximal scallop, was deployed in the aorta. Bridging stents to the innominate and left common carotid arteries were used to seal the aneurysm. The left common carotid to left subclavian artery bypass was used to revascularise the covered native subclavian artery.
Technical success was achieved and a postoperative CT angiography at one month showed exclusion of the aneurysm with open left subclavian artery and without bird-beak configuration.
Treatment of a chronic type B aortic dissection with Valiant Captivia after ascending aorta and arch surgery
Luigi Lovato (Bologna, Italy) presented the case of a 73-year-old female with a history of hypertension, ascending aorta surgery for aneurysm and chronic type B dissection from middle descending thoracic aorta. The patient was submitted to arch aneurysm surgical replacement. Serial CT imaging showed progressive severe dilation (>5mm/year) of aortic isthmus and middle thoracic aorta false lumen, joining 5.5cm at the level of the big entry tear with appearance of partial false lumen thrombosis.
The patient was considered for TEVAR of both aortic lesions. Lovato said that challenges arose due to the surgical reconstruction anatomy with angulation between the prosthesis and native aneurysmatic aorta and difficult visualisation of the reimplanted epiaortic vessel, creating a relatively short proximal neck, and discrepancy between the proximal and distal neck, because the sinusoidal intimal flap and the relatively distal entry tear forced them to cover the whole thoracic aorta extending next to the coeliac trunk where the true lumen is small.
He said that the Valiant Captivia (Medtronic) stent graft was selected because “it combines a solid capture tip system to obtain a high controlled stent deployment and low risk of wind-sock and bear-beck effect and, at the same time, an optimal hydrophilic delivery system to manage the advancement through a relatively small femoro-iliac access and tortuous aorta”.
During the procedure, Lovato explained, they adopted a different approach, delivering the distal stent graft first to solve the neck discrepancy. The second stent was deployed afterwards, proximally, in the surgical prosthesis and with the distal part inside the first distal stent. The sizing was sorted to achieve the best oversizing in the prosthesis, avoiding type I/III endoleak.
The pre-discharge CT control (six days later) showed an optimal stent graft positioning without type I/III endoleak. A small type II endoleak was visible at isthmic level. There was a moderate retrograde flow from the abdominal false lumen reaching the middle thoracic aorta, but only in the late CT scan.
“The case demonstrates the need for careful planning to meet the anatomical challenges in patients with previous arch surgical reconstructions and the feasibility and safety of this ‘distal first approach’ of TEVAR for type B dissection. This technique can solve the frequent neck discrepancy of these patients avoiding the severe complication of distal false lumen aneurysmal degeneration, caused by the intimal flap rupture induced by the stent graft radial forces,” Lovato concluded.
ZENITH Alpha thoracic endograft
Geert Schurink (Maastricht, The Netherlands) reviewed the case of an 80-year-old female whose thoracic aneurysm was discovered after she underwent a computed tomography (CT) scan for a deep vein thrombosis. He said she was “a very fit” 80-year-old but did have a kyphosis and moderate femoral pulses.
The patient underwent TEVAR with the Zenith Alpha thoracic endograft (Cook Medical). Afterwards, in the recovery unit, mean arterial pressure was >85mmHg and a cerebral spinal fluid drain was not put in because of her concerns about her kyphosis. However, two days after the procedure, she lost sensation in both lower legs and experienced weakness in her left leg. A cerebral spinal fluid drain was put in and her mean arterial pressure was >90mmHg. Schurink reported that the patient made an “almost complete recovery”. He added that he did not believe the loss of sensation was a result of an embolization (in the distal aorta) because if it had been, he said, she would not have made a recovery. Furthermore, there were no signs of emboli on angiography.
After the case was presented, the audience not only discussed when to put in a cerebral spinal drain after TEVAR but also reviewed whether patients were allowed to get too hypotensive compared with their relative baseline pressure.
Nellix – Endovascular aneurysm sealing (EVAS) with polymer in the aortic sac
In the case that Michel Reijnen (Arnhem, The Netherlands) reported, a 70-year-old male patient, with a cardiopulmonary history, presented with an infrarenal aneurysm with a maximum diameter of 55mm.
The patient was treated using the Nellix endovascular aneurysm sealing system (Endologix). Reijnen explained that Nellix consists of two balloon-expandable endoframes surrounded by endobags that are filled in situ with a biostable polyethylene glycol (PEG)-based polymer to completely fill the aneurysm sac, thus sealing the entire aneurysm.
The case showed that the infrarenal neck diameter and length were respectively 28mm and 35mm. Access was adequate and the calculated volume to seal the aneurysm was 64ml. After a bilateral cutdown, an angiography was performed with a calibrated catheter. Subsequently, two Nellix endosystems were inserted, positioned and deployed. A prefill with saline solution, to a pressure of 180mmHg, was performed showing a complete exclusion of the aneurysm. The saline solution was then replaced by 55mL of polymer to reach a final pressure of 190mmHg. During curing the Nellix balloons were re-inflated.
Completion angiography showed a complete exclusion of the aneurysm with patent renal and hypogastric arteries. Throughout one year follow-up, there were no complications.
Reijnen advised that the “key points for success are case planning and extensive knowledge of the device and technique, which differs from regular EVAR”.
The INCRAFT low profile device introduced percutaneously
A patient with a 55mm aortic abdominal aneurysm was the subject of the case that Hany Zayed, Gianbattista Parlani and Jost Philipp Schäfer (London, UK; Perugia, Italy; and Kiel, Germany, respectively) presented. They reported on how the patient was treated with an Incraft endograft (Cordis).
The procedure was performed under local anaesthesia with a bilateral percutaneous approach. The presented case showed the endograft’s main body delivery and the possibility to reposition the graft, as well as the Iliac limbs implantation accommodating the device into the broad spectrum of maximum and minimum overlapping in order to obtain the correct length of the device to preserve the hypogastric artery.
The completion angiography performed at the end of the procedure showed precise endograft placement with the start of the covered portion of the device immediately below the lowest renal artery and total coverage of both common iliac arteries with preservation of the hypogastric artery.
Parlani noted that the key message of the presentation “is that this ultra-low profile device is designed for proximal and distal placement accuracy and allows for customisation during the procedure to accommodate a wide range of anatomical sizes. This broad anatomical coverage is offered with a minimal number of product codes for easier pre-procedural planning”.
Challenging AAA anatomy treated by Endurant IIs stent graft
Medtronic’s Endurant IIs stent graft has several advantages and benefits for managing patients with challenging anatomy, Johannes Gahlen (Ludwigsburg, Germany) said in his case presentation of a 64-year-old male with an abdominal aortic aneurysm of 5.2cm.
He explained that the device has expanded anatomical customised options, including a 20% reduction in distal diameter compared with select Endurant II bifurcation stent grafts, in-situ sizing with select ipsiliateral limbs, allowing a 3–5 stent overlap for adjustment curing cases, and easier pre-case planning to simplify sizing. It has also has a “better anatomical fit” with “less material encumbrance in tight aortic bifurcations and “better precision in distal landing to avoid type IIb endoleak or unintended coverage of the hypogastric artery.
Gahlen reported that, in the case he was presenting, endovascular aortic aneurysm repair was performed with the graft using the three-piece approach. He described the patient’s anatomy as challenging because there was kinking and stenosis in the left common iliac artery and the right common iliac artery was dilated.
According to Gahlen, the usual practice is to perform the follow-up CT scan at six months, but three-month follow-up was planned for this patient because the device was relatively new.
The Aorfix endovascular stent graft
The patient in the case presentation from Robert Beasley (Miami, USA) was an 81-year-old male with a history of diabetes, hypertension, chronic obstructive pulmonary disease, coronary artery disease, and smoking (he quit in 2009). Beasley commented that the patient was found to have a 7.8 x 7.3 x 10cm abdominal aortic aneurysm with an angle of
He explained that the patient was treated with an Aorfix endovascular stent graft (Lombard). Features of this device include, according to Beasley, radioplaque markers on the stent body graft and legs for “precise positioning”, 8mm sealing zones at proximal distal ends of stent grafts, and a “fishmouth” fixation mechanism (the proximal end of the graft expands into a fishmouth shape). Beasley called this mechanism a “unique design concept” that allows the endograft to be placed transrenally and has four pairs of hooks to “resist migration forces”.
The E-liac device in complex iliac aneurysm
In his presentation, Jost Philipp Schaefer (Kiel, Germany) explained that a 56-year-old male patient developed a dissected iliac aneurysm in the left common iliac artery after undergoing surgical repair of an aortoiliac aneurysm with an aortofemoral (right) and aortoiliac (left) graft interposition.
The dissection caused a severe stenosis at the ostium of the internal iliac artery with symptoms of buttock claudication and erectile dysfunction. Via combined left groin and transbrachial approaches, the dissected iliac aneurysm is repaired by utilising the E-liac device and an E-ventus stent graft (Jotec) as bridging stent. The applicable techniques were demonstrated step-by-step, including the completely percutaneous approach under local anaesthesia, the successive deployment of the iliac-side-branch graft, and the placement of the bridging stent graft.
Schäfer said that this Edited Live Case “demonstrates the technique of endovascular repair of iliac artery aneurysm with the new E-liac device. Available off-the-shelf, the E-liac device is a low-profile iliac-side-branch graft, offering a percutaneous treatment option for a wide range of aortoiliac aneurysmatic pathologies”.
Control of infection with prosthetic grafts: added value of INTEGARD SYNERGY
Jean-Paul de Vries (Nieuwegein, The Netherlands), in his case presentation, explained how the Intergard Synergy (Maquet) graft was used to treat a 62-year-old female with (as identified on CT scan) a mycotic abdominal aorta combined with Leriche syndrome. He explained that the graft was the only one to be loaded with two different antiseptics, adding that these antiseptics (silver acetate and triclosan) are “not limited to one target and do not have the likelihood to produce bacterial resistance”. De Vries added that the graft was “designed for routine prophylactic use” and was not associated with any contraindications for contaminated implant sites.
In this case, the patient was on the hospital ward for 12 days after the operation and received penicillin for eight weeks afterwards. According to de Vries, there was no sign of infection at the two month postoperative CT scan.
Hello world!
cxsymposium2016-10-12T13:01:18+01:00Welcome to WordPress. This is your first post. Edit or delete it, then start blogging!
“Ask the expert” workshop dedicated to imaging
cxsymposium2016-10-12T13:01:18+01:00To complement the CX Imaging Day morning session, in the afternoon delegates will have the opportunity to learn about new imaging systems in a hands-on workshop including different imaging modalities used in vascular and endovascular management.
The “Ask the expert” workshop will be held at the GE Healthcare, Hansen Medical, Philips Healthcare and Siemens imaging stands in the Exhibition Hall. Physician-experts in imaging will be giving demonstrations combined with talks focusing on sizing, 2D/3D fusion imaging and how to achieve optimal dose levels.
Imaging industry stands programmes
The programmes at the imaging companies’ stands will combine hands-on demonstrations and talks. They will run from 1:30pm until 5:30pm on Thursday 30 April.
GE Healthcare booth 130
Faculty/demonstrators: Stéphan Haulon, Lille, France and Adrien Hertault, Lille, France
- EVAR procedures: From sizing to fusion
- Achieving optimal dose levels
Hansen Medical booth 221
Programme to be announced
Philips Healthcare booth 318
Faculty/demonstrators: Frank Vermassen, Gent, Belgium and Jim Reekers, Amsterdam, Netherlands
- Vessel Navigator: Learn all about the latest innovation in Live Image Guidance solutions
- 2D Perfusion: Get insight in the latest perfusion angiography solution
- Veradius Unity: Experience the innovative vascular workflow of our newest Mobile C-arm with flat detector
- How to build a Hybrid room: Considerations when purchasing a Hybrid room
Siemens booth 420
Faculty/demonstrators: Dittmar Böckler, Heidelberg, Germany (second speaker TBA)
- Adding smooth to smart in EVAR guidance
- Learn about innovative tools to treat complex endovascular aortic repair cases with confidence
- The new PURE Software for the Artis zeego enables a smooth workflow to use the innovative smart tools at the day of operation
CX Imaging Day: High-quality imaging is the key to optimal results
cxsymposium2016-10-12T13:01:18+01:00Stéphan Haulon, Richard McWilliams and Alan Lumsden, Faculty members of the CX Imaging Day, new style Parallel Session of the Charing Cross Symposium 2015), outline the relevance of therapeutic imaging for vascular and endovascular procedures, the use of robotics and radiation exposure concerns. These topics will be discussed at the CX Imaging Day on Thursday 30 April.
This year, the CX Imaging Day will provide a comprehensive programme on state-of-the-art imaging applied for the intervention of the aorta, carotid arteries, peripheral arteries and veins, involving planning and using hybrid theatres, robotics, 3D fusion and guidance, intravascular ultrasound (IVUS), perfusion angiography, cone beam computed tomography (CT) and carbon dioxide angiography. In the morning, attendees will hear the latest data on high-quality imaging and be able to discuss the different applications. During the afternoon, the “Ask the expert” workshop is organised in the Exhibition Hall with visits to the industry stands, focusing on sizing, 2D/3D fusion imaging and how to achieve optimal dose levels.
Stéphan Haulon (Aortic Centre, Hôpital Cardiologique, CHRU Lille, France), says delegates will get insights from large cohort published studies to identify the various strengths and limitations of high-end imaging. He adds: “We will report the latest progress, provide an update on radiation baseline for various procedures, and see how these new imaging techniques can be extended to peripheral limbs.”
Richard McWilliams (Radiology Department, Royal Liverpool University Hospital, Liverpool, UK) notes that the immediate and continuing success of endovascular interventions relies heavily on medical imaging. He comments: “The processing power of modern imaging workstations allows the physician to rapidly assimilate the relevant anatomy before intervention. Procedural success is facilitated by the quality of modern hybrid suites and the associated software that accompanies these allowing 3D imaging, 3D fusion and 3D guidance. Surveillance after endovascular treatment is now of higher quality because of improvements in CT scanners and ultrasound techniques including contrast-enhanced ultrasound. Direct comparison of one CT after aneurysm repair with a prior CT is now a joy when these are registered and locked together on dual monitors and reviewed in cine mode.”
Haulon states that the rise of catheter-based procedures and minimally invasive surgery has required concomitant advances in intraoperative imaging applications, and that hybrid rooms have made this possible by combining the mobility and sterility of an optimal open surgical environment with the latest advanced imaging capabilities of a fixed system such as image fusion or cone beam CT. “Experience has shown that the routine use of advanced imaging techniques, such as fusion, during endovascular aneurysm repair has significantly reduced the radiation exposure to patients and operators, as well as the contrast volume load, without jeopardising the overall procedure workflow,” he says.
The role of intraoperative imaging will be also highlighted in the Main Programme – Abdominal Aortic Controversies session (Wednesday 29 April) with a presentation by Dittmar Boeckler on intraoperative CT in ruptured abdominal aortic aneurysm treatment with EVAR.
The portability of imaging (eg. ultrasound) and the more widespread availability of CT and MRI scans have transformed diagnosis and now the treatment of vascular disease, according to Alan Lumsden (Houston Methodist Hospital, Houston, USA). “We are in an era where portability makes imaging technology available in the operating room—image-guided surgery and endovascular surgery. This can be either by direct imaging or increasingly the use of image fusion methodologies.
At the CX Imaging Day, Lumsden will show how the use of robotics can be beneficial for different procedures—carotid, complex cases and even retrieval of inferior vena cava filters. “Navigation through the vascular system depends on the use of wires, catheters and a series of wall interactions with these devices. Such techniques have not fundamentally changed in decades. Catheter robotics promises a level of catheter and wire control that simply cannot be achieved using traditional techniques,” he says. Lumsden explains that his group’s interest in robotics was generated by watching an electrophysiologist take the robotic catheter through the inter-atrial septum, in a beating heart, while applying measured pressure and radiofrequency energy at multiple points, under precise control to the wall of the left atrium. He adds, “It was hard not to be impressed with these capabilities, hence our interest in the peripheral application of robotics. We believe that controlled navigation, stability and pushability of the Magellan catheter (Hansen Medical) will permit us to perform procedures which cannot be completed using manual techniques. Further it will allow us to steer devices which lack sterility (snares, thrombectomy devices, atherectomy catheters), thereby improving their capabilities.”
Haulon will present a new integrated workflow for sizing, 2D/3D fusion and assessment of endovascular aneurysm repair (EVAR). He explains: “Time is crucial, improving vascular surgeon sizing and fusion workflow is key, so why not preparing both steps at the same time? Completion contrast-enhanced cone beam CT has also demonstrated its capacity to assess technical success, reducing the requirement for a postoperative CT angiography and allowing accurate detection of complications requiring additional treatment right on the spot. Also, there is a need to consider radiation dose at a higher level than that of the procedure itself… we need to take into consideration the patient’s dose throughout the entire treatment process, from diagnosis, to treatment and including follow-up. Having the right dose management tools in place allows me to achieve this.” Giuseppe Pannuccio (Muenster, Germany) will be also presenting on the role of 2D/3D fusion imaging in complex aortic procedures.
3D imaging will be further explored by Tara Mastracci (London,UK) who will discuss the role of 3D guidance in fenestrated endovascular aneurysm repair (FEVAR) and by Frank Vermassen (Ghent, Belgium) who will show how 3D imaging can be used in carotid procedures. Jan Brunkwall (Cologne, Germany) will focus on 3D imaging in complex aortic procedures.
Radiation exposure concerns
Radiation protection remains a major concern and will be one the topics discussed at the CX Imaging Day, and new technology is helping to change behaviour and lower doses, says McWilliams, and adds: “Live dose monitoring provides instant feedback to operators and assistants so that they are aware how their actions increase or decrease dose. Newer equipment is increasingly being designed with dose-saving in mind. Intravascular ultrasound during venous procedures is an important part of this.”
He maintains that “we must also remember the simple things that reduce dose which I see forgotten very commonly. These include increasing the distance from the X-ray source, using face screens, using pump injections rather than hand-injections to allow the operator to increase his/her distance, reducing the pulse rate and frame rate and knowing the relevant projections from the preoperative dataset rather than by trial and error during the procedure. An unfortunate reality is that although modern equipment is capable of exquisite fluoroscopic images, the operator needs to ensure that the images are good enough but not needlessly better than this, to avoid excessive dose to the patient and staff. If the image quality is too good then this is bad”.
Radiation exposure will be also highlighted in the Main Programme – Abdominal Aortic Controversies session with a mini-symposium dedicated to explore radiation reduction strategies for the operator and the patient, including a presentation on dose optimised imaging protocols for complex endovascular procedures by Eric Verhoeven (Nurnberg, Germany). In the Peripheral Arterial Controversies session (Tuesday 28 April) of the Main Programme, Martin Malina (Malmo, Sweden) will be speaking about contrast reduction angiography for peripheral arterial disease.
In the afternoon, delegates will visit the Exhibition Hall (GE Healthcare, Hansen Medical, Philips and Siemens booths) for technology demonstrations.
The New CX Imaging Day will take place at the Charing Cross Symposium on Thursday 30 April – Olympia Room Learning Centre in the morning and Exhibition Hall in the afternoon, Olympia Grand, London, UK.
Click here to see the CX Main Programme Sessions
Click here to see the CX Imaging Day
Click here to register
Vascular malformations and limb ischaemia: Main topics of the CX Paediatric Vascular Issues session 2015
cxsymposium2015-07-11T02:23:23+01:00In 2015, the CX Paediatric Vascular Issues session will host presentations on modern management of childhood vascular disorders with a special focus on vascular malformations and limb ischaemia as well as other pathologies (aortic and arterial). Course directors, Malcolm Simms (Birmingham, UK) and George Hamilton (London, UK), outline the hottest controversies in the field.
Simms says that there are at least three areas of controversy when treating children with vascular issues, “the appropriate management of iatrogenic acute limb ischaemia, the optimal management of vascular malformations and the tailoring of vascular intervention in children to address issues of growth”.
Adding to the current controversies in the field, Hamilton comments: “There is a very low level of training or at the very least understanding of available treatments and protocols in current vascular training programmes throughout Europe. As a result poor treatment and outcomes are common.” He adds, “Paediatric vascular treatment should be delivered in specialist children’s centres.” However, there is still a need to develop such centres to deliver optimal care, he notes.
With regards to improvements in the field, Simms states that the most relevant advance has been the development of smaller calibre angiography catheters and devices. “This has extended the scope of endovascular diagnosis and intervention to smaller children.” Hamilton comments that another relevant advancement is the “growing expertise and confidence in the use of thrombolysis in acute ischaemia with the huge potential to avoid amputation and save limbs.” The understanding of vascular malformations has also been improved with better nomenclature and classification, Simms adds.
The course directors note that acute limb ischaemia treatment is the area that urges most attention in paediatric vascular treatment. Even though there are some advances in the field, there is still a special need to further develop (particularly for children younger than five) small diameter endovascular catheters, wires and absorbable small diameter stents, which seem to offer the best potential for treatment because of growth issues, Simms and Hamilton comment. Simms adds that the management of large diffuse vascular malformations remains “palliative at best”.
Delegates who attend the CX Paediatric Vascular Issues session will learn about the latest treatment methods in the field and will also have the chance to discuss specific examples with case-based presentations.
The CX Paediatric Vascular Issues session will take place at the Charing Cross Symposium on Thursday 30 April (morning) – London Room Learning Centre, Olympia Grand, London, UK.
Click here to see the CX Main Programme Sessions
Click here to see the CX Paediatric Vascular Issues session
Click here to register
Call for case submissions to the CX Paediatric Vascular Issues session
cxsymposium2015-07-11T02:23:23+01:00The Charing Cross Symposium is looking for paediatric vascular management dilemmas to encourage audience discussion at the CX Paediatric Vascular Issues session.
Clinicians are invited to submit paediatric cases concerning acute or chronic limb ischaemia, trauma, mid-aortic syndrome, cancer resection and vascular malformations.
Format: Cases should be submitted in Powerpoint (maximum three slides or the equivalent to a three-minute presentation). The material should be limited to the case presentation for discussion by the delegates and expert panel – no ‘lectures’ or literature review of the associated condition should be included.
Deadline for submission: 17 April 2015
Please send your cases to: Katherine Pole ([email protected])
Get the latest update on vascular malformations management
cxsymposium2015-07-11T02:23:23+01:00Delegates attending the CX Vascular Malformations Management session on Thursday 30 April, in the afternoon, will get a general overview of congenital vascular malformations and will also learn about the latest advancements in the field.
This year, the course will include keynote lectures covering the topics of venous, lymphatic, combined and arteriovenous malformations each followed by case presentations illustrating representative clinical situations. The role of imaging of vascular malformations and treatment options including embolisation, sclerotherapy and laser therapy will be addressed.
The session, led by Iris Baumgartner, Bern, Switzerland, will be interactive allowing the audience to get feedback from a panel, which includes the experts: Raul Matassi, Matthias Widmer, Joe Brookes, Andreas Saleh and Martin Köcher.
The CX Vascular Malformations Management session will take place at the Charing Cross Symposium on Thursday 30 April (afternoon) – London Room Learning Centre, Olympia Grand, London, UK.
Click here to see the CX Main Programme Sessions
Click here to see the CX Vascular Malformations Management session
Click here to register
Four days of Venous content to be run for the first time at Charing Cross
cxsymposium2016-10-12T13:01:18+01:00In 2015, the Charing Cross Symposium will dedicate, for the first time, four days of discussion in the venous field addressing both the superficial and deep venous systems. The Symposium is also launching a new CX Venous Workshop, formerly named as CX Office-Based Vein Practice Course, which includes hands-on training on the latest techniques for varicose veins treatment and, for the first time, practical training on deep venous treatment.
On the first day (Tuesday, 28 April) upcoming leading vascular and endovascular experts will present cutting-edge venous investigation at a CX Abstracts‒Venous session. Delegates will also have the chance to visit the CX Venous Workshop, which will run on the following days (Wednesday, 29 April and Thursday, 30 April). On the fourth day, the CX Main Programme will be dedicated to Venous Controversies.
Superficial and deep venous treatment at the core of the Venous Controversies
This year’s Venous Controversies in varicose veins treatment are no longer between surgery and office-based techniques, according to Roger Greenhalgh, (Imperial College, London, UK), chairman of the CX Programme Organising Board. “It seems as if office-based vein techniques have swept the board. Now the controversy seems to be about which method to select in which situation,” he says.
Delegates will learn the latest evidence on varicose veins treatment options with thermal and non-thermal techniques. “We will try to present a balance between data and information that relate to office-based tested techniques that are already established and are probably state-of-the-art alongside techniques that are emerging, and that may not have enough data but nonetheless might represent a completely shift in practice. We are happy for people to find out about these new techniques at Charing Cross first,” says Ian Franklin (London, UK), member of the CX Programme Organising Board.
Commenting on non-thermal techniques, Mark Whiteley (University of Surrey, London, UK), also a member of the CX Programme Organising Board, says: “The newer non-thermal techniques are now joining the endovenous thermal ablative devices and starting to show impressive results. The controversy here will be whether these newer non-thermal methods are now able to replace endothermal ablation in some or all cases.” (See an interview with Whiteley on varicose veins treatment).
Sclerotherapy and laser therapy of cosmetic skin problems will also be discussed.
Deep venous treatment is another major topic of controversy at this year’s CX Symposium. Franklin comments: “Deep venous thrombosis treatment is controversial because it is extremely badly managed in many countries and some people get almost no treatment at all. Nowadays, there are new technologies that help physicians treat deep venous thrombosis effectively. There is a lot to achieve in terms of training and education on how to diagnose deep venous thrombosis promptly with the right method of treatment to guarantee durable results.”
Stephen Black (Guy’s and St Thomas NHS Foundation Trust, London, UK), member of the CX Programme Organising Board, considers that there is a real need to develop the evidence base for treatment options for deep venous thrombosis. At the session on Deep Venous Controversies, delegates will learn about the latest evidence on the current techniques for deep venous thrombosis treatment including new stents and catheter-directed thrombolysis and thrombectomy techniques. (See an interview with Black on deep venous disease treatment).
Imaging for superficial and deep venous treatment is playing a vital role in the diagnosis of these anomalies. Greenhalgh comments: “It appears that imaging is absolutely at the core of intervention in the superficial and deep venous systems. Unfortunately, venous imaging is less than helpful when the patient lies flat and how to image the deep venous system with the patient standing will be discussed.”
The use of current imaging technologies such as duplex, magnetic resonance imaging (MRI) and intravascular ultrasound (IVUS) will be discussed at a session dedicated to this field.
New CX Venous Workshop
The former CX Office-Based Vein Practice Course has changed its name to the CX Venous Workshop because it now encompasses all aspects of venous disease including deep vein thrombosis, intravascular ultrasound and deep vein stenting. The course will continue providing delegates with hands-on training sessions for varicose veins treatment. In 2014, it had a record attendance of over 700 delegates during two days.
The CX Venous Workshop is set out in a very flexible format and will run from 10am until 3pm over two days. There will be different training stations (around 30) led by a physician expert who will be demonstrating a wide range of techniques and procedures in different superficial and deep venous treatment techniques. There is not a rigid timetable and participants can choose their timing at their convenience. The course facilitates one-to-one interaction or discussions in small groups.
Key areas covered include:
- Practical training
- Truncal ablation
- Diagnostic venous ultrasound
- Anatomy
- Venous haemodynamics
- Sclerotherapy
- Thread veins
- Dermal lasers
- Pelvic venous imaging
- Pelvic congestion
- Pelvic veins and haemorrhoids
- IVUS
- Deep vein stenting
- Pelvic vein embolisation
- Lymphoedema
- Endovenous glue
- Mechanochemoablation
- Steam
- Wound care
- Varicose veins
- Perforators
- Tributaries
- Surgery
- Caval filters
- Intermittent pneumatic compression
- Stockings and bandaging
- Neuromuscular electrical stimulation
- Thrombophilia and DVT Venous malformations
The CX Venous Controversies Day will take place at the Charing Cross Symposium on Friday 1 May – Main Auditorium, Olympia Grand, London, UK.
Delegates will have the opportunity to get hands-on training on the latest techniques for superficial and deep venous system treatment at the CX Venous Workshop on Wednesday 29 April and Thursday 30 April – Gallery Upper Level, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to see the CX Venous Workshop
Click here to register
Insights into the current practice for varicose veins treatment
cxsymposium2016-10-12T13:01:18+01:00Venous surgeons and phlebologists are spoiled for choice when it comes to office-based technology options for varicose veins. This brings questions such as what technologies are best suited for what patients, what factors should be considered to obtain best results and what is the role of open surgery in the field. Mark Whiteley (University of Surrey, London, UK), member of the CX Programme Organising Board, gives his views on the subject. The latest evidence in this area will be discussed in the Venous Controversies sessions of this year’s CX Main Programme.
Physicians now have a great selection of office-based technologies for the treatment of varicose veins; what method is best for what case?
This has been the major area of my research since starting radiofrequency ablation in March 1999 and it is fair to say that there are many different opinions on this. Most importantly, before this question can even be considered, we need to decide on what constitutes “the best”. In my view, technical perfection is the correct outcome, as this will lead to long-term success and therefore should lead to long-term patient improvement.
However, advocates of techniques with inferior technical results that might require less intervention or fewer injections, point to patient satisfaction as the correct outcome measure to use to assess “the best” treatment method. I believe this is not a good outcome measure to use scientifically. Patient satisfaction can be manipulated by factors other than the treatment itself, particularly by misinformation given to patients in some studies by advocates of one method as to the theoretical risks of other methods that might be used.
In addition, satisfaction has to be measured at one or several points in time—often in the relatively short term. Technical imperfections which are likely to progress to recurrence and reduced patient satisfaction in the long term might be present in the short term. But measuring patient satisfaction alone may miss these out due to the time it takes for the patients to experience the clinical outcomes of the treatment failures.
What factors should be considered to obtain best results in varicose veins treatment?
As with all surgical techniques, there are several factors involved in obtaining the best results. Not only does the technology and device design have to be adequate to the job, but the patient selection needs to provide appropriate cases for the chosen technique and doctors and their teams need to use adequate imaging and procedural technique to get the optimal results.
In addition, patient expectation has to be added, such as whether they are looking for relief of symptoms only or cosmetic perfection and also an acceptable cost for the service.
What office-based technologies are currently showing best results?
Generally, in most hands, endovenous thermoablation shows excellent results for truncal and perforator veins ablation, provided the correct technique is used and an appropriate device for the vein to be treated. Glue and mechanochemical endovenous ablation (MOCA) are starting to show promising results in truncal venous reflux in selected patients whereas foam sclerotherapy is technical disappointing in truncal veins. However, advocates will point to patient satisfaction and re-treatments to argue for its use.
Currently, coil embolisation of refluxing pelvic veins with foam sclerotherapy seems to be the optimal treatment for pelvic venous reflux. Foam sclerotherapy alone or ligation of veins at the pelvic exit points are used by some but need to be proven as effective and acceptable treatments.
For incompetent tributaries and varices, ambulatory phlebectomy is probably optimal for larger veins and foam sclerotherapy for smaller veins— although the new technique of combining both as “foam phlebotomies” is gaining popularity in the USA.
When it comes to neovascular tissue, strip tract revascularisation, feeding veins under patches of telangiectasia and primary avalvular varicose anomalies (PAVA) then foam sclerotherapy is the only effective option.
Finally, for telangiectasia themselves, microsclerotherapy is still the best option as it treats both the visible veins as well as the communicating veins hidden below the surface.
With the outcome of many office-based technologies and their comparable efficacy results with surgery, is there still room for surgery?
No.
Unless one calls ambulatory phlebectomies (surgery), then there is no need to ever perform open surgery for varicose veins anymore. Personally, I have not had to use open surgery for any varicose veins case for over a decade.
At the debate “Duplex scanning is mandatory before treatment of asymptomatic cosmetic thread veins” you will present “For the motion”. Could you tell us the reasons as to why you are supporting this practice?
Since 2001, increasing numbers of research papers have shown that cosmetic thread veins frequently occur in association with underlying venous reflux. In many, if not the majority of cases, this venous reflux leads directly into the thread veins. Furthermore, every case of the complication called telangiectatic matting that I have ever seen after thread vein treatment, has an underlying untreated vein refluxing into the affected patch.
Since instituting a policy of duplex scanning in 2002 in which every patient presenting with cosmetic thread veins of the legs are treated for any underlying venous reflux before proceeding to microsclerotherapy of the thread veins themselves, we have never had a case of telangiectatic matting. Those cases presenting to us with this complication from elsewhere invariably have an underlying vein refluxing into the lesion.
With the explosion of cosmetic clinics starting to offer thread vein treatments, often without duplex ultrasound or even hand held Doppler, it is essential for us as phlebologists and venous surgeons to state that we feel that treatment of thread veins without a duplex ultrasound scan first is a sub-optimal treatment.
The CX Venous Controversies Day will take place at the Charing Cross Symposium on Friday 1 May – Main Auditorium, Olympia Grand, London, UK.
Delegates will have the opportunity to get hands-on training on the latest techniques for varicose veins treatment at the CX Venous Workshop on Wednesday 29 April and Thursday 30 April – Gallery Upper Level, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to see the CX Venous Workshop
Click here to register
Specifically designed stents and imaging: Key players in deep venous treatment
cxsymposium2016-10-12T13:01:18+01:00The development of new stents specifically designed for deep venous reconstruction, new techniques for lysis and imaging are playing a key role in the treatment of deep vein thrombosis; an area that, according to Stephen Black (Guy’s and St Thomas NHS Foundation Trust, London, UK), member of the CX Programme Organising Board, has been largely neglected for years. In this interview, Black, who will be chairing a session dedicated to the deep venous system at the Venous Controversies session of CX 2015, speaks about the advances in the field.
What is the major controversy in deep venous treatment?
The main controversy is still the role of the new venous stents and whether these will really change treatment and outcome for patients with chronic obstructive disease causing post thrombotic syndrome.
What methods are showing optimal results in deep venous reconstruction?
Currently the new techniques for lysis and the development of more robust stents have renewed interest in this area. There is certainly great potential with these new developments; however, we still need to be cautious using them given the real absence of descent data to support how, when and why we treat these patients.
Now more stents have been developed specially for the venous anatomy; what impact have these devices had in the treatment of venous disease?
The new stents for venous treatment have raised awareness and led to a renewed focus on what was a largely neglected area for a number of years. Patients with symptoms after deep vein thrombosis are desperate for treatment and have received the answer of nothing to be done for too long. As a group of clinicians we need to embrace this momentum to see if we can make a difference to this group of patients.
What is the role of imaging in deep venous interventions?
The role of imaging is to try and outline anatomy in the best possible manner. In particular, we need to identify what inflow the patient will have into the area that needs stenting and also whether there is a problem more proximally. It helps to identify if there is an obstructive lesion (i.e. May Thurners/Cockets lesion) and whether the patient may require a purely stent procedure or possibly open surgery or a combination of both.
What imaging methods are showing best results?
Intravascular ultrasound (IVUS) and magnetic resonance imaging (MRI) currently have the greatest potential for diagnosis in deep venous treatment. MRI helps to reduce the radiation dose in what are usually a young group of patients. MRI has also the potential to age clot, which is really exciting (delegates will have the opportunity to learn about this MRI capability at the Venous Controversies of the CX Main Programme). IVUS may also help reduce radiation exposure but also provides significantly more real time detail when treating these patients.
Currently we are limited by the imaging modalities all providing us with static images. We need to develop techniques that help identify what the flow is like in the system and measure pressure.
The CX Venous Controversies Day will take place at the Charing Cross Symposium on Friday 1 May – Main Auditorium, Olympia Grand, London, UK.
Delegates will have the opportunity to get hands-on training on the latest techniques for deep venous thrombosis treatment at the CX Venous Workshop on Wednesday 29 April and Thursday 30 April – Gallery Upper Level, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to see the CX Venous Workshop
Click here to register
Best management of short abdominal aortic aneurysm necks to be highlighted in the CX Abdominal Aortic sessions
cxsymposium2016-10-12T13:01:18+01:00The controversy around the treatment of infrarenal abdominal aortic aneurysm necks of less than 15mm will spark the discussion in the Abdominal Aortic Controversies Day of this year’s CX Main Programme. Delegates will also hear the latest evidence on the best way to manage a ruptured aneurysm and will have the opportunity to discuss whether too many patients with ruptured abdominal aortic aneurysms are being denied intervention. In addition, the controversy regarding radiation damage to the operator and the patient will be addressed and a series of clinical approaches for elective aneurysm repair will be discussed.
“The management of the aortic neck of less than 15mm is certainly very controversial,” says Roger Greenhalgh (Imperial College, London, UK), chairman of the CX Programme Organising Board. Therefore, he comments, “We will try to address every possible way of treatment.”
Commenting on endovascular aneurysm repair (EVAR) for short necks, Andrew Holden (Auckland City Hospital, Auckland, New Zealand), member of the CX Programme Organising Board, says: “We are aware that using EVAR to treat infrarenal aneurysms with hostile necks has been historically associated with higher aneurysm-related morbidity and mortality. Can new EVAR technologies and techniques change this paradigm?”
Delegates will have the opportunity to hear about the latest evidence regarding EVAR, open surgery and new technologies that are addressing the treatment of infrarenal abdominal aortic neck at the CX Abdominal Aortic Controversies Day (Wednesday 29 April). (Read an interview on the subject with Frans Moll, co-chairman of the CX Programme Organising Board).
Management of ruptured abdominal aortic aneurysms
The latest evidence on the best way to manage a ruptured aneurysm will be presented including the one-year results of the IMPROVE trial and the IPD 3 trial. In the same session, the controversy as to whether many patients with ruptured abdominal aortic aneurysms are denied intervention will be discussed.
Greenhalgh comments: “Perhaps doctors are becoming more concerned about operating for fear of having poor mortality figures. We need to find evidence whether indeed patients are denied intervention.”
Janet Powell (Imperial College, London, UK), member of the CX Programme Organising Board, considers that a few patients are offered repair of ruptured aortic aneurysm because perhaps “not all centres have an endovascular team and facilities available at all times; additionally, there are financial stringencies on healthcare systems and many of these patients may need intervention out of hours.”
Holden adds that many questions on this subject remain unanswered: “Do we have sufficient data to support an ‘EVAR first’ approach for ruptured abdominal aortic aneurysm repair patients? If not, who should be offered EVAR? Does the lower early morbidity associated with EVAR mean more ruptured aneurysms should be treated?”
These and other questions will stir a debate on the subject to be led by Matt Thompson (St George’s Vascular Institute, London, UK) and Peter Lamont (North Briston, NHS Trust, Bristol, UK).
Radiation exposure concerns
Additionally, in the CX Abdominal Aortic Controversies Day, strategies for radiation exposure reduction to the operator and to the patient will be discussed in a mini-symposium. Members of the CX Programme Organising Board consider this subject a top priority in the field. Greenhalgh comments: “The risk of radiation damage to the operator and to the patient is now known to be serious and many physicians have damaged themselves trying to treat their patients.” Powell adds: “Many of the early pioneers of EVAR/TEVAR are now dying of cancer. The importance of reducing radiation burden and safety is one of the key contemporary issues that need to be addressed.”
Subsequently, delegates will learn about a series of clinical approaches for elective aneurysm repair including the latest evidence on a new iliac branch endoprosthesis and treatment strategies for octo- and nonagenarians.
Moreover, the long-term follow-up of EVAR after 20 years of introduction will be also addressed. Holden comments: “The optimum follow-up protocol after EVAR is also likely to be controversial. This is an important discussion as post-procedural surveillance is a major cost contributor to EVAR.”
The CX Abdominal Aortic Day will close with a mini-symposium on the management of type I endoleaks.
The CX Abdominal Aortic Controversies Day will take place at the Charing Cross Symposium on Wednesday 29 April – Main Auditorium, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to register
Frans Moll speaks on the management of short abdominal aortic aneurysm necks
cxsymposium2015-07-11T02:23:23+01:00Frans Moll (University Medical Center Utrecht, Utrecht, The Netherlands), co-chairman of the CX Programme Organising Board, gives his views as to why the management of short necks is a hot topic in the field and discusses the current strategies of treatment. He will moderate the session “Procedures for infrarenal abdominal aortic neck” of the CX Abdominal Aortic Controversies Day.
Why is the management of aortic necks of less than 15mm controversial?
There is still an axiom that durability for proximal fixation of the aortic neck with endovascular aneurysm repair (EVAR) depends on sealing and fixation, so you do not only need fixation but you also need a certain amount of sealing zone. The critical sealing zone corresponds to one ring of the stent graft. This is usually between 1.2 and 1.5cm in length.
What are the factors that must be considered when treating short infrarenal aortic necks with endovascular approaches?
If you think that the patient will really benefit from endovascular procedures as opposed to open procedures you need to accept shorter necks (>1.5cm in length). The conditions in which it can be accepted are either suprarenal fixation or T-branched.
What endovascular approaches are currently addressing the management of this type of necks?
In shorter stenting we could use sealing prototypes; for example, there is a device that inflates the sealing zone and another one where the polymer is used to fill the aneurysm sac. You could also use a stent graft that is specifically designed for shorter necks (1.5cm up to 1cm). There is also a system that uses endoluminal staples designed to secure the neck.
At the “Procedures for infrarenal abdominal aortic neck” session, physicians will debate whether EVAR is not sensible for abdominal aortic aneurysms with a neck length shorter than 10mm; are you for or against the motion and why?
I think that necks shorter than 10mm can be treated safely with EVAR but you need to consider the latest generation stent grafts. Not every stent graft is able to provide a good result between 8mm and 10mm length.
The CX Abdominal Aortic Controversies Day will take place at the Charing Cross Symposium on Wednesday 29 April – Main Auditorium, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to register
Management of type B aortic dissection tops agenda in the CX Thoracic Aortic sessions
cxsymposium2016-10-12T13:01:18+01:00Type B dissection of the aorta will be the main topic of controversy at this year’s Charing Cross Symposium. Predictions of clinical success after thoracic endovascular aortic repair (TEVAR), factors that predict success after TEVAR for chronic type B dissection, the latest evidence for embolisation of false lumen and spinal cord protection are some of the topics that will be discussed in the CX Thoracic Aortic Controversies Day (Thursday 30 April).
Last year, the majority of delegates who attended a debate titled “For uncomplicated type B dissections, early intervention is indicated” voted against the motion. This year, the debate in this area will assess whether the long-term results following intervention for sub-acute uncomplicated type B dissection warrant intervention.
The Thoracic Aortic Day will also include discussions on the incidence of stroke during aortic arch interventions, treatment options for ascending and arch of the aorta including TEVAR, branched stent grafts for complex arch lesions, open surgery and the use of robotic systems.
Furthermore, amongst the descending thoracic aortic, thoracoabdominal and juxtarenal aortic controversies, the question of threshold diameter for intervention in the thoracic aorta will be raised. Roger Greenhalgh (Imperial College, London, UK), chairman of the CX Programme Organising Board, says: “Evidence for the precise diameter to intervene is extremely sparse and this will be brought out in the session”.
Interview with Andrew Holden, member of the CX Programme Organising Board
Andrew Holden (Auckland City Hospital, Auckland, New Zealand), member of the CX Programme Organising Board and chairman of the session “Controversies in chronic type B dissection of the aorta” speaks on the subject.
Why is the management of chronic type B dissections controversial?
Currently, this is one of the most important sessions at CX and hopefully some consensus will emerge. There is no doubt that treatment of acute complicated type B aortic dissection is widely accepted. What is less clear is whether uncomplicated type B dissection should undergo endovascular treatment and if so when (acute, sub-acute or chronic). In chronic type B dissection, the biggest challenge is to successfully exclude the aneurysmal component (preventing retrograde false lumen perfusion) and maintain branch artery patency.
What are the factors that must be considered when treating chronic type B dissections?
The common thread when treating chronic type B dissection is to exclude the primary (or inflow) intimal tear. It is then unclear how much aorta needs to be covered and how retrograde perfusion of the false lumen can be avoided. In the dissected and aneurysmal abdominal aorta, the challenge is to exclude the false lumen but maintain branch artery patency.
What challenges in TEVAR have to be overcome in order to avoid complications?
Feared complications in TEVAR include retrograde dissection and spinal ischaemia. A number of strategies have been developed and will be discussed at the CX Thoracic Aortic Controversies Day.
The CX Thoracic Aortic Controversies Day will take place at the Charing Cross Symposium on Thursday 30 April – Main Auditorium, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to register
New CX Edited Live Cases session focuses on aortic and carotid procedures
cxsymposium2016-10-12T13:01:21+01:00With the aim to study key controversies from its Main Programme in technical detail, the Charing Cross Symposium is launching the CX Edited Live Cases, a session that will focus on aortic and carotid procedures. Delegates will have the opportunity to explore the application of different techniques in complex situations, which perhaps could not have been broadcast in a live case because of patient’s safety. Roger Greenhalgh (Imperial College, London, UK), chairman of the CX Programme Organising Board, explains the details of this new session.
Why are the CX Edited Live Cases focusing on the aortic and carotid fields?
The CX Programme Organising Board decided that live cases will be broadcast only in situations where the patient’s life is not at risk and where the surgeon is doing something which is not life-threatening, hence the existence of the CX Live Peripheral Arterial Cases. In peripheral procedures the risk is not as high as in aortic or carotid interventions so we thought it was legitimate to have live cases only for lower limb procedures.
The CX Edited Live Cases are being offered in situations in which the patient’s life or condition could in some way be made worse through having the operator concentrate on filming rather than focusing on the patient. In aortic procedures the slightest slip could be very disastrous for the patient. In this kind of intervention the surgeon requires complete concentration on the patient. Another example is in carotid procedures where there is a risk of embolisation to the brain.
What makes the CX Edited Live Cases different from edited live cases presented at other conferences?
The difference in our Edited Live Cases is that we are relating this session systematically to our Main Programme (Aortic and Carotid) and at all points we will be stressing the evidence that the procedure works. For example, a short presentation in the Main Programme could include a specific device for abdominal aortic aneurysm treatment. In the CX Edited Live Cases that same product can be exposed to show in more detail what is special about it, compared to other aortic devices, and how and why it can be used in that specific case. The topics in the Main Programme normally give the evidence on how to fix a certain condition, but there will always be discussions about why it is thought it works and for the audience to cross question any person. The CX Edited Live Cases will bring an additional space to expand on these discussions and show the technical details.
What kind of procedures should delegates expect to see in the CX Edited Live Cases session?
Delegates should expect to see cases addressing endovascular aneurysm repair (EVAR), thoracic endovascular aortic repair (TEVAR) and endovascular aneurysm sealing (EVAS). The cases will include thoracic complex procedures and abdominal procedures of different types. A carotid mesh procedure, which is aimed to reduce embolisation to the brain to prevent stroke, will also be presented. In addition, we are going to have an Edited Live Case about how to control infection, which you could not do in a live case. We will have approximately 10 Edited Live Cases.
Could you explain what the format of the session will be?
Similar to the Main Programme sessions, the panel of the CX Edited Live Cases will include a chairman, the operator in the Edited Live Case, the phycisian who will be explaining it to the audience and two invited discussants. The speaker will present the case including patient characteristics, reason for the procedure, the necessary investigation undertaken (eg. X-rays), the choice of devices available, the strategies of treatment proposed and all of the pitfalls and concerns raised before the procedure.
After this presentation, a 10-minute edited video will be presented. In order to allow room for discussion about a specific technique the video could be stopped—this is an added benefit you could not have in a live case presentation. This approach will help to improve the educational basis of this session. After that, the aim will be to engage the audience in discussion.
Delegates will also have the opportunity to see these cases post Charing Cross 2015 from a video library that will be created from this initiative.
The CX Edited Live Cases (Aortic and Carotid) will take place at the Charing Cross Symposium on Tuesday 28 April – Grey Learning Centre, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to register
Superficial femoral artery at the centre of the CX Peripheral Arterial sessions
cxsymposium2016-10-12T13:01:21+01:00Strategies for treatment of the superficial femoral artery with the concepts of “Leaving nothing behind” or “Leaving something in” will take centre stage in the Peripheral Arterial Controversies session of this year’s CX Main Programme. In addition, delegates will hear about early clinical outcomes with a new flexible endoluminal stent for iliac artery disease. The treatment of popliteal aneurysms by endovascular means will also be subject of discussion, as well as the best treatment options for below-the-knee lesions.
Roger Greenhalgh, chairman of the CX Programme Organising Board, says that the session aiming at “unpacking” the superficial femoral artery will be one of the highlights of the CX Peripheral Arterial Day in the Main Programme (Tuesday 28 April). “We will try to inform the audience about what to do in the superficial femoral artery and when, depending on the type and length of lesion and based on the available evidence.” He adds, “The speakers will consider more severe arterial disease which in their view requires a stent. If something is to be ‘left behind’ this needs to be justified”.
Commenting on the “Leaving nothing behind” concept, Cliff Shearman (University of Southampton, Southampton, UK), member of the CX Organising Board, says: “This is a great idea but the technology (drug-coated balloons, atherectomy) is expensive and we have to see the evidence on how much benefit it does give and for how long.”
Moreover, attendees will learn the results, at five years, of the superficial femoral artery treatment with open surgery.
Delegates will also have the opportunity to discuss in more detail the superficial femoral artery controversies on Thursday 30 April in two roundtables to be held at the CX ilegx Collaboration Day course.
Furthermore, in the CX Peripheral Arterial Day, iliac reconstruction will be addressed with early clinical outcomes of a new flexible endoluminal stent graft. Additionally, the treatment of popliteal aneurysms by endovascular means will be subject of discussion. Commenting on his view on the topic, Shearman says: “Although this is commonly advocated we need to see the long-term results. Also we need to ask ourselves, ‘How many popliteal aneurysms are really suitable for endovascular treatment’?” Physicians will have the chance to discuss their views during this debate at the end of the session.
Below-the-knee treatment options and their effectiveness will also be discussed. Michael Edmonds, member of the CX Organising Board, says: “Addressing below-the-knee intervention strategies is of great importance considering the dramatic rise in the incidence of diabetes mellitus.” Greenhalgh adds: “The below-the-knee arteries are smaller and have difficult technical challenges—the durability of the procedures is seldom stated longer than for two years.”
When treating patients with peripheral arterial disease Greenhalgh says, “advising the patient on self-management including smoking cessation, exercise and healthy living is fundamental”. The second principle, he adds, is to intervene only when it is necessary and avoid making the patient worse. And finally, it is necessary to intervene with a technique as minimal as possible and that can produce durable results. “In the end, it is the patient’s quality of life that matters and if the arterial disease is overcome the symptoms should improve,” he notes.
The CX Peripheral Arterial Day will take place at the Charing Cross Symposium on Tuesday 28 April – Main Auditorium, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to learn more about ilegx
Click here to register
CX ilegx Collaboration Day: Key strategies to save limbs
cxsymposium2019-10-07T14:47:14+01:00The CX ilegx Collaboration Day course has been designed to update attendees on the latest treatment strategies developed to avoid the increasing number of unnecessary lower limb amputations—flagship principles of the ilegx initiative. Delegates will learn about the King’s College Hospital open access vascular diabetic foot care pathway—an effective approach which incorporates early diabetic foot referral and interdisciplinary work—which is showing promising results saving limbs.
Delegates will also hear about revascularisation strategies of the ischaemic foot with the latest data in the field; and will have the opportunity to discuss in more detail, in two roundtables led by experts, the controversy of “Leaving something in” or “Leaving nothing behind” in the superficial femoral artery. This subject will be exposed in the CX Main Programme – Peripheral Arterial Controversies (two days before ilegx).
In the commentary below, Michael Edmonds (King’s College Hospital, London, UK), who is one of the founders of ilegx and co-director of the CX ilegx Collaboration Day course, explains the King’s College Hospital pathway. He also writes about the value of endovascular techniques for diabetic foot care and critical limb ischaemia treatment.
This is followed by an interview with Cliff Shearman (University of Southampton, Southampton, UK), co-director of the ilegx Collaboration Day. He talks about what he believes the take-home message from this year’s CX ilegx Collaboration Day will be.
Commentary by Michael Edmonds, ilegx course director
Early referral, fast track care and multidisciplinary work: Key approach to save limbs
The ilegx Collaboration Day will include an account of a modern successful approach to the diabetic ischaemic foot in the King’s College Hospital (London, UK) open access vascular diabetic foot care pathway, which has resulted in greater than 90% limb salvage rate.
This approach is based on a new understanding of the natural history that has led to a novel classification of the ischaemic diabetic foot, emphasising the importance of the diabetic neuroischaemic foot as well as the critically ischaemic foot. This pathway is operated by an interdisciplinary team comprising surgeon, physician, podiatrist, nurse and orthotist and provides integrated care focused on a diabetic foot clinic. The diabetic foot can deteriorate with alarming speed and for this reason the clinic provides open rapid access to accelerate urgent assessment and to proceed quickly to state-of-the-art interventions in the revascularisation of not only the critically ischaemic foot but also the neuroischaemic foot. This includes prompt decision making within the interdisciplinary team as to proceed to endovascular or open vascular surgery (or both in a hybrid technique), involving revascularisation of both legs and increasingly, pedal arteries. The diabetic neuroischaemic foot is particularly characterised by ulceration and complicating infection and within this interdisciplinary diabetic foot service, modern techniques in wound care and aggressive treatment of infection with surgical debridement and parenteral antibiotics are also important.
The value of endovascular techniques for diabetic foot care and critical limb ischaemia treatment
There is a crucial role for endovascular techniques to revascularise the foot, whether it is to restore blood flow in order to help diabetic ulcers to heal, or longer-term as a treatment for critical limb ischaemia.
It is agreed that the critically ischaemic foot should be urgently revascularised so as to save the limb either by endovascular procedures or open vascular surgery. However, controversy exists when there is a diabetic patient with a neuroischaemic foot ulcer that is not healing in a moderately ischaemic limb. These patients are often not getting the benefit of endovascular procedures in a timely fashion.
Although the neuroischaemic foot would not have got into trouble unless it had been subjected to minor trauma—which is often unsensed because of nerve damage—it is important to understand that, having got into trouble, the ulcer cannot be healed because the blood supply to the foot cannot be increased. Thus there is a crucial role for below-the-knee endovascular procedures to improve the blood supply, even if it is a temporary increase, to get such ulcers healed. Once such ulcers are healed it will not matter if subsequently there is restenosis.
Interview with Cliff Shearman, ilegx course director
In this year’s CX iLegx Collaboration Day, what do you think the take home message will be?
Shearman: Avoiding amputation is relatively easy; it is about early treatment and not ignoring the problem. The biggest barrier to success is poor organisation and lack of awareness of the seriousness of the problem. Solving the problem improves outcomes, improves quality of care and saves a lot of money.
This year’s ilegx roundtables will discuss revascularisation strategies in the superficial femoral artery; do you believe you should “Leave nothing behind” or “Leave something in”?
Shearman: Better understanding of the biology of diabetic vascular disease will lead to better therapies. While it is appealing to consider therapies, which do not leave anything behind, we have to understand what the main effect (short- and medium-term) of an intervention is on the plaque to design the optimum therapy.
About ilegx
The ilegx initiative, launched in 2008, was created in response to the increasing number of lower limb amputations which are mostly due to type II diabetes.
ilegx is a collaboration of like-minded health professionals, patients and care workers who share the view that too many legs are amputated and many of these are completely unnecessary.
The ilegx mission is to attract attention and draw awareness to the need for an improvement in health care in order to lower unnecessary major amputation of legs.
The CX ilegx Collaboration Day will take place at the Charing Cross Symposium on Thursday 30 April – Grey Learning Centre, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to register
CX Live Peripheral Arterial Cases to be broadcast for the first time at the CX Symposium
cxsymposium2016-10-12T13:01:21+01:00This year, the Charing Cross Symposium is launching the CX Live Case method. Thomas Zeller (Universitäts-Herzzentrum Freiburg – Bad Krozingen, Bad Krozingen, Germany), course director of the CX Live Peripheral Arterial Cases, will link the live case demonstrations from Bad Krozingen with topics to be discussed in the CX Main Programme – Peripheral Arterial Controversies at the Charing Cross Symposium 2015.
Starting with shorter and simpler lesions, Zeller and his team will aim to demonstrate the value of drug-coated balloons against plain old balloon angioplasty. Beyond this, longer and also calcified arterial stenoses will be tackled. The audience will see which stents are selected, and whether atherectomy pre-stent is thought to be beneficial. Zeller will show which stent devices can reach longer lesions and into the popliteal artery, and which require pre-treatment with adjunctive therapies. The value of drug-eluting stents will also be demonstrated, and the management of in-stent restenosis and its correction by mechanical means will be shown.
In this interview, he explains the value of live case presentations at conferences and the importance of relating the CX Live Peripheral Arterial Cases to the topics of the CX Main Programme – Peripheral Arterial Controversies, and encourages audience interaction in this session.
What are the benefits of including live case presentations in medical conferences?
Live case presentations—provided they are unbiased—support the transfer of clinical science into clinical practice. New technologies and treatment algorithms can be demonstrated (supplementing talks and roundtable discussions) and the potential limitations and advantages in daily routine can be highlighted. With live case presentations; conference audience gets a better and practical impression of how new technologies can be implemented in the interventionalist’s routine.
What are the benefits of having the CX Live Peripheral Arterial Cases relate to what is discussed in the CX Main Programme – Peripheral Arterial Controversies?
The attendee gets immediate insights into the clinical application and potential pitfalls of new interventional strategies, which have been discussed in the CX Main Peripheral Programme (The CX Main Programme – Peripheral Arterial Controversies will take place on 28 April 2015, a day before the CX Live Peripheral Arterial Cases). Every new technology imposes a learning curve which might become shortened if the attendee gets advice from experienced operators who have already gained experience in the application of interventional strategies or use of those new technologies.
The live cases for the Charing Cross Symposium have been selected based on the topic of a given session to enable the panel and audience to discuss these topics with the operators.
The CX Main Programme – Peripheral Arterial Controversies dedicates a session to the discussion of revascularisation strategies in the superficial femoral artery; which key controversies will you try to shed light on with the CX Live Peripheral Arterial Cases session in this field?
In the CX Peripheral Live Cases, the main issue of controversy to discuss is the potential role of drug-coated balloons and drug-eluting stents for the treatment of the superficial femoral artery. We will analyse whether (or not) these devices are worth the costs compared to standard treatment (plain old balloon angioplasty and bare metal stenting). Moreover, the role of spot stenting in a drug-coated balloon setting will be discussed and finally the potential role of vessel preparation prior to the use of drug-eluting technologies.
What techniques and technologies will be demonstrated at the CX Live Peripheral Arterial Cases session?
We will show interventions dealing with drug-coated balloons and provisional stenting, stenting of kink zones, vessel preparation with debulking devices, how to treat in-stent reocclusions, etc.
The CX Symposium advocates strong audience interaction; how will you encourage participants’ interaction in the CX Live Peripheral Arterial Cases session?
Audience interaction has to be pushed by the moderators and panellists. We as operators will be open to questions from the audience and we will also invite the audience to express their own experiences with the interventional techniques, which will be shown during the live case transmissions.
The CX Live Peripheral Arterial Cases session will take place at the Charing Cross Symposium on Wednesday 29 April – Grey Learning Centre, Olympia Grand, London, UK
Click here to see the CX Main Programme Sessions
Click here to see the CX Parallel Sessions
Click here to register
Intravascular imaging important to avoid rupture after blunt aortic injury
cxsymposium2016-10-12T13:01:21+01:00Benjamin Starnes (Seattle, USA) told the audience attending yesterday’s mini symposium on acute aortic transection that intravascular ultrasound (IVUS) was an important tool in the management of patients with blunt aortic injury undergoing thoracic endovascular aortic repair (TEVAR) because it helps to ensure accurate sizing of the endograft and prevent rupture. The session also reviewed the differences between image-based classifications of blunt aortic injury.
Starnes reported that, over the past decade, endovascular repair of patients with blunt traumatic aortic injury has become the “predominant approach to fixing these injuries”, but added that there were “unique challenges” to using the endovascular approach. He said: “One of challenges is the dynamic nature of the aorta. There are some interesting data from blood-letting studies in Yorkshire pigs that show significant decrease in aortic diameter accompanying induced haemorrhagic shock, with a dose-dependent effect.”
According to Starnes, aortic diameter is also known to increase after resuscitation and the area with the greatest change in size is the area that is most commonly injured in blunt trauma (ie. the isthmus and descending aorta). He added: “CT angiography is used for diagnosis of these injuries and axial slices from the initial scan are often used for planning and sizing of the repair. While using IVUS at the time of repair to better characterise the injury, we noticed a difference in the aortic diameter with systolic variation [compared with the diameter observed on the initial CT angiography]. This then begs the questions “Is initial CT angiography appropriate for sizing the endograft in these patients?” and “Is that endograft going to be undersized once that patient is fully resuscitated?”
Starnes and colleagues, therefore, conducted a retrospective chart review of patients with blunt aortic injury who underwent TEVAR at their level-one trauma centre to determine if there was a difference between the aortic diameter observed on diagnostic CT angiography and that observed on IVUS at the time of the repair. The inclusion criteria were initial admission or pre-admission CT angiography, IVUS at the time of repair, and a post-implant CT angiography. Of the 26 patients who were treated at the centre during the study period (July 2007–July 2011), three patients did not have post-implant CT angiography information, one patient was converted to surgery, and six patients did not receive IVUS—leaving 16 patients available for assessment.
There was a significant difference of 2.4mm between mean aortic diameter with initial CT angiography and mean aortic diameter with IVUS: 21.7mm vs. 24mm, respectively (p=0.004). Starnes noted: “When we look at post-implant CT angiography compared with initial CT angiography, there is again a highly significant difference of 3mm (p=0.0001). But, when we compared post-implant CT angiography with IVUS, there was no difference.” He added that when they reviewed theoretical graft diameters, there was a significant difference of 2.4mm (p=0.003) between the size of the graft that would be chosen based on the initial CT angiography and that based on IVUS at time of repair. There was also a significant difference in graft size between post-implant CT angiography and initial CT angiography (3mm; p=0.0002), but no difference in size between post-implant CT angiography and IVUS.
“I believe IVUS is a critical and important tool for the management of patients with blunt aortic injury,” Starnes concluded.
Ali Azizzadeh (Houston, USA) also presented data at the mini symposium, reviewing the long-term effects of intentional stent graft coverage of the left subclavian artery (in patients with blunt aortic injury undergoing TEVAR). He said that in a review of 82 patients undergoing TEVAR at his centre between September 2005 and July 2012, 50 received intentional stent graft coverage of their left subclavian artery. Azizzadeh reported: “Intentional coverage of the left subclavian artery during TEVAR for blunt aortic injury appears safe without compromising mental or physical health outcomes. Furthermore, left artery stent coverage does not increase the long-term risk of upper extremity symptoms or impairment of normal activities.”
Does intramural haematoma exist?
As well as presenting data for the role of IVUS in the management of patients with aortic, Starnes also reviewed the classification of the injury. He reported that he and his colleagues developed a new image-based classification system for these types of injury because they believed the current classification system, by the Society for Vascular Surgery (SVS), was “lacking”. Starnes explained: “The SVS system has four grades of injury—grade one, intimal tear; grade two, intramural haematoma; grade three, pseudoaneurysm; and grade four, rupture—but does not provide for any treatment recommendations because grades one to three are treated the same and we do not believe grade two exists. In a review of 140 aortic transections at our centre, we did not see a single case of intramural haematoma.”
Therefore, they developed a new classification system based on the presence or absence of an aortic external contour abnormality. Under this new system, intimal tear (intimal defect and/or thrombus of
However, Michael Dake (Stanford, USA) commented that there was a lack of consensus surrounding intramural haematoma as unlike Starnes and colleagues, Rabin and colleagues did recognise the existence of intramural haematoma and classified it as being a “grade one” injury (in contrast to the SVS classification). He added that Osgood and colleagues, in an another system, classed intramural haematoma as being “grade 1B” but he noted that they did not find any patients with that type of injury in their series. Dake reported, regardless of how it was classified, recent publications have suggested that: “Intramural haematoma without associated peri-aortic component, contour abnormality, or pseudoaneurysm may be conservatively managed with appropriate follow-up imaging.”
CX audience unconvinced about early intervention for uncomplicated type B dissections
cxsymposium2016-10-12T13:01:21+01:00The CX Great Debate saw Peter Taylor, London, UK, and Richard Gibbs, Imperial College, London, UK, persuaded 71% of the audience at the Charing Cross International Symposium yesterday to vote for their position against the motion “For uncomplicated type B dissections, early intervention is indicated”.
Their opponents in the debate, Christoph Nienaber, Rostock, Germany, and Jan Brunkwall, Köln, Germany, had the support of 29% of the audience. Audience discussion at the end of the session identified a need for a larger randomised trial with long-term follow-up that had clinically meaningful endpoints.
Nienaber and Brunkwall set out to persuade the audience that placing a stent graft early was important in order to heal the aorta. “Rupture, malperfusion, hypertension and down the line, aneurysm formation are some of the risks of type B dissection,” Brunkwall said.
Nienaber made the point that scaffolding was the only way to stabilise the progression of the dissection as data available so far showed that the disease is characterised by a downhill evolution over time. “If there is no scaffold, and the aorta is not remodelled, you are confronted with ongoing attrition rate in terms of cardiovascular death and progression of the disease. This is beneficial for even in so called stable or clinically silent patients,” he said. He made his views clear that no dissection was ever uncomplicated.
“Only pre-emptive stenting will ensure long-term remodelling and stability. Only remodelling stabilises the aorta and no drug that induces remodelling, only the likelihood of rupture, so supportive scaffolding should be offered to any type B dissection,” Nienaber said.
“What do we achieve with TEVAR in the setting of type B acute dissections? Fewer later interventions and lower mortality after five years,” Brunkwall said. The five-year long-term follow-up of the INSTEAD XL showed a statistically significant reduction in aorta-specific mortality and a statistically significant reduction in disease progression in favour of the combined TEVAR plus medical therapy arm.
Brunkwall also made the point that the ADSORB trial with false lumen thrombosis as an endpoint, had shown that patients receiving TEVAR have significantly more complete and partial thrombosis than the ones getting best medical therapy.
The ADSORB trial was a prospective randomised trial to compare TEVAR with best medical therapy in patients with acute uncomplicated type B aortic dissection. At the follow-up for one year, there was no death stroke or paraplegia in either group at 30 days and aortic remodelling at one year favoured the intervention but it did not reach statistical significance.
Taylor and Gibbs based their arguments around the fact that medical treatment is getting better. Briggs noted that a recent study (Fattori et al. J Am Coll Cardiol 2013;61:1661-78) had shown that survival at five years without intervention is 70–89%. They also highlighted the fact that TEVAR carries risks including retrograde dissection; stroke (3%); paraplegia (2.5%) and other complications. The team stated that all current endografts used in dissection are designed for use in aneurysms.
“There is no adequate evidence to recommend early intervention in all but a few patients with uncomplicated type B dissection. Only a small subgroup at high risk of aortic expansion will benefit from early intervention and these patients can be identified using techniques such as functional imaging,” Briggs said
Taylor also attacked the quality of evidence from randomised controlled trials that supported early intervention. “The ADSORB trial had only 61 patients in total. It was underpowered and stopped at one year and was therefore too short and small to answer questions about survival and efficacy. The INSTEAD trial had only 140 patients in total and underpowered for survival. In total, there have been only 201 dissection patients ever randomised,” he said.
Taylor made the point that drug therapy was getting better and that the available evidence showed that the majority of patients are alive with medical treatment; there is 80% survival at five years and that therefore, medical treatment was safe for the majority of patients with uncomplicated type B dissection. They do not need early intervention and there is an early mortality associated with TEVAR that cannot be ignored, he said.
A member of the audience noted that in order to make a clinical difference, all-cause mortality has to be the only endpoint at five years and the trial should be designed to show superiority. Martin Bjorck, Uppsala, Sweden, also commented on the difficulty faced in every day clinical practice. “There is a problem because we know from the data from the two randomised trials that it is rather safe to do TEVAR, but that there is a 3–5% risk of very serious neurological complications that cannot be discounted. We know from the long-term results of the INSTEAD trial that if you do not treat, you have many patients developing dilatations and needing treatment later on that is more complicated. We need a larger randomised trial with long-term follow-up.”
CX voting results
Earlier in the session, 65% of the CX audience had voted that registry data is now irrelevant and that it was time that randomised trial evidence for TEVAR dissection became available. Nearly 80% also believed that aortic intervention for acute dissection should only be performed in recognised vascular centres with cardiothoracic surgery on site.
Early results from subclavian artery branched endoprosthesis studies presented at CX
cxsymposium2015-07-11T02:23:23+01:00Michael Dake, Stanford, USA, outlined the first experiences with the Gore TAG (W L Gore) thoracic branch endoprosthesis and Frank R Arko, Charlotte, USA, presented on the MONA LSA (Medtronic) branched device results at a session yesterday. Both speakers shared early cases and their results.
Thoracic aortic aneurysms that involve the left subclavian artery often leave physicians no choice but to use surgical techniques or to cover the branch vessel. “Although reports from single-centre experience with the hybrid approach have been positive, a single branch thoracic endograft specifically designed for treatment of the aortic arch (Zone 0–2) could be useful in extending the advantages of endovascular repair to the aortic arch. As such, the Gore TAG Thoracic Branch Endoprosthesis is designed as a modular system which allows for treatment of aortic arch pathologies using a less invasive hybrid endovascular approach,” Dake said.
He presented on the Zone 2 US investigational device exemption feasibility trial that will enrol 20 to 40 patients at six sites. Patients will be followed for five years. The study will evaluate the device for the treatment zone 2 aneurysms.
The Gore TAG thoracic branch endoprosthesis has a modular construction design with off-the-shelf components. It has an inner lumen for anchoring and sealing the branch component. The complete system consists of aortic and branch components designed for the use in the arch, and also the accompanying accessory devices to facilitate delivery.
“The system is easy to use with a single femoral access and requires minimal catheter manoeuvres. It is safe with zero ischaemic time and has low risk of branch vessel coverage. After the first four cases, successful access and deployment of the endoprosthesis was seen in all cases. There was one procedural type 1 endoleak that resolved without re-intervention at one month. There were no device-related endoleaks, but one type 2 endoleak was seen at one month. There were no deaths or neurological events. There were no site reported serious adverse events related to the device,” he said.
Dake explained that the procedure to deploy the device included inserting the guidewires in aorta and branch vessel; introducing the aortic component over both guidewires into position within the arch; deploying aortic component and withdrawing catheter; advancing the introducer sheath and dilator and advancing and deploying branch component.
In order to be enrolled, patients had to have descending thoracic aortic aneurysms requiring placement of the proximal extent of the aortic stent graft in Zone 2. The primary endpoints of the study were: successful access and deployment of the thoracic branched endograft and the primary patency of the side branch assessed by angiography when the procedure finishes. The secondary endpoints included a one-month core lab analysis, assessing the side branch primary patency and device-related endoleaks.
Arko told delegates that there was a clinical need for left subclavian artery preservation in association with encroaching thoracic artery aneurysm. He presented the current status of an early feasibility trial in the FDA’s new innovation pathway testing the Valiant Mona LSA device.
The key goals of the trial are to validate the procedure in humans; assess the safety and performance acutely and at 30 days and collect imaging data to augment the current understanding.
Arko told delegates that the Valiant Mona LSA Thoracic Stent Graft consisted of a flexible cuff “volcano” on the main body. The system is a two-graft system and the main graft system comes in diameters between 30 and 46mm and in the single length of 15cm. It is a two-wire system as well and the secondary wire can be snared and then the second branch is brought up and deployed. The branch graft itself is made of a nitinol helical wire and polyester fabric. It has a proximal flare. The branch graft is always 40mm in length and a 15F profile, femoral access system.
The early feasibility trial is a prospective, non-randomised, three-centre, premarket clinical study that has enrolled nine patients. The primary safety and effectiveness objectives were measured acutely and at 30 days. “The follow-up schedule will be at 0-30 days, six months, 12 months and annually through five years,” Arko said.
The principal investigators are Eric E Roselli, Cleveland, USA, Frank R Arko, Charlotte, USA and Matt Thompson, London, UK.
Arko revealed that the current status of the early feasibility trial had seven patients enrolled as part of the US cohort and two enrolled as part of the cohort outside the US. One emergent case had been enrolled in the USA, outside of trial. Acute procedure results from all seven patients revealed 100% technical success and 100% patency in both main and branch stent graft. There were no type I or III endoleaks.
“All current devices need a healthy landing zone to seal. To achieve sufficient landing zone, the left subclavian artery may be sacrificed with resulting complications, such as 6% arm ischaemia, 4% spinal cord ischaemia, 2% vertebrobasilar ischaemia, 5% anterior circulation stroke and 6% death. Left subclavian artery preservation is recommended in the literature. 17–43% of patients undergoing TEVAR have planned coverage of the left subclavian artery to achieve an adequate proximal seal and the coverage of the left subclavian artery without revascularisation is the single most important predictor of post-TEVAR stroke,” Arko said.
CX ilegx Collaboration Day hosts Endovascular Electronic Education and focuses on key approaches to save limbs
cxsymposium2016-10-12T13:01:21+01:00At the ilegx Collaboration Day, attendees learnt about the best management therapies for diabetic foot care and vascular reconstruction in critical limb ischaemia patients and for the second year running, the symposium broadcast edited live cases treating superficial femoral artery lesions to the Far East and North America in an event named Endovascular Electronic Education, sponsored by Abbott Vascular.
In the morning, three edited live cases were showcased to the Far East. The first case was performed by Andrej Schmidt in Leipzig, Germany, the second procedure was carried out by Peter Goverde in Antwerp, Belgium, and the third case was performed by Josef Tacke in Passau, Germany. The Abbott Supera stent system was used to treat occlusion and total occlusion of the superficial femoral artery and proximal popliteal artery.
Schmidt, Goverde and Tacke were present to answer questions from the audience at CX and those watching the live broadcast in the Far East.
Responding to questions about how a physician chooses a stent, Schmidt said: “In some cases it is clear—if it is a calcified lesion, for me it is clear that a drug-eluting balloon might not be so good, we know this from our data, and the Supera stent is the stent of choice.” He added that more data are required to make the stent selection process more comprehensive.
In the afternoon, edited live cases were broadcast to North America.
Early referral, fast track care and multidisciplinary work: key approach to save limbs
The ilegx initiative, launched in 2008, was created in response to the increasing number of lower limb amputations which are mostly due to type II diabetes. Michael Edmonds (London, UK) who is one of the founders of ilegx, introduced the “diabetic foot care” session, which was organised in conjunction with the King’s College Hospital Open Access System, with a presentation which highlighted the importance of early diabetic foot referral and interdisciplinary work as an effective approach to reduce the number of lower limb amputations, flagship principles of the ilegx initiative. He said: “Up until recently, the diabetic ischaemic foot has defeated every health care system in the world. However, a strategy which combines early referral and interdisciplinary working has led to improvements in care.” With this in mind, he commented, “ulcers can now be healed and amputations can be prevented.”
Edmonds also referred to the importance of organising a “fast-track” service in a “one-stop” visit, comprising clinical assessment, same-day investigations and urgent management to treat infection and revascularise the foot, when dealing with cases of “diabetic foot attack.” “This is best carried out in a diabetic foot clinic which can see the ischaemic patient in an open access system without delay and has rapid availability of debridement and intravenous antibiotics to treat infection and control the septic vasculitis.” He also said that diabetic foot patients who experience repeated crises from the rapid onset of infection need a special form of such easily accessible care provided by the diabetic foot clinic. “Such integrated fast track care can rapidly restore the circulation, limit tissue necrosis, save the limb from amputation and provide complete recovery from the foot attack,” he commented.
An interdisciplinary team including podiatrists, nurses, orthotists, microbiologists, interventional radiologists and surgeons—including vascular surgeons, orthopaedic surgeons and plastic surgeons—is the ideal team required in the management of diabetic foot, Edmonds noted.
He also mentioned that to complete an effective management of diabetic foot, follow-up and rehabilitation are required.
Interdisciplinary views on diabetic foot care
A vascular surgeon, an interventional radiologist and a podiatrist from King’s College Hospital, London, UK, shared with CX delegates their best practices treating diabetic foot.
Vascular surgeon Hisham Rashid, said that “the incidence of type II diabetes mellitus is increasing across the world with an expected rise in 2030 to more than 12% in a large population of the world.”
This is a worrying figure taking into account that “a major amputation rate is significantly higher in diabetic patients,” he noted.
Rashid told CX delegates that aggressive revascularisation with angioplasty, bypass or hybrid techniques is essential for limb salvage and reduction of major amputation rate. He mentioned that at King’s College Hospital, 77% of cases are treated with angioplasty and 23% with distal bypass. He said that distal bypass surgery “plays a major role in revascularisation, especially in patients presenting with significant tissue loss and when angioplasty is not feasible.” Hybrid techniques in “no-option” critical leg ischaemia have also proven very effective in preventing major amputation in this challenging group of patients, he commented.
At King’s, Rashid noted, revascularisation using distal and ultra-distal bypass has a very good outcome with a one-year amputation rate of 3.4% and 30-days mortality rate of 1.5–1.7%. At one-year, “mortality is significantly influenced by end-stage renal failure and age rather than diabetes mellitus,” he said.
Interventional radiologist Dean Huang, said that the concept of “foot attack” and “time is tissue” in diabetic patients means that treatment of an infected ulcer should be handled as an emergency with the management of a multidisciplinary team. At King’s College Hospital, “we follow this approach; we work on the basis of rapid access to diagnosis, rapid access to intervention and follow-up of interventional and surgical procedures.” From the interventional radiologist perspective, he said, “rapid access to imaging diagnostics enables the selection and planning of the optimal strategy.” Ultrasound, CTA and MRA have their place and angiography remains the gold standard, he commented. “Prompt definitive treatment with radiological intervention and/or surgical bypass to revascularisation for healing in conjunction with wound care and antibiotics is the key to achieve prevention of amputation,” Huang noted. “The threshold of what can be treated with endovascular procedures is shifting as more sophisticated devices appear on the market.”
Podiatrist Jennifer Tremlet, spoke about the different techniques used at King’s College Hospital to heal diabetic foot wounds. “Diabetic foot patients are complex cases, they experience extensive tissue loss and infection. In order to achieve successful wound healing, they require intensive wound care and rigorous monitoring.” She said that, depending on the complexity of the wound and the type of patient, they use different multi-modal techniques including debridement, larvae therapy, hydrosurgery therapy topical negative pressure therapy, split skin grafts and pressure relief.
Foot care and arterial reconstruction
At a session on foot care and arterial reconstruction, Christopher Attinger (Washington, USA) said that using the angiosome principle to guide revascularisation is a critical component for optimising wound healing. He explained that the angiosome concept divides anatomic regions into three-dimensional blocks of tissue fed by source arteries and creates a framework for understanding tissue perfusion, predicting wound healing, and planning surgical interventions. Therefore, “understanding the boundaries of an angiosome and the vascular connections between source arteries provides the basis for limb salvage to optimise revascularisation to ischaemic areas and promote wound healing.”
Mauro Gargiulo (Bologna, Italy) spoke on the need to have multidisciplinary guidelines to support the treatment of critical limb ischaemia. He referred to a consensus document that has been recently published on the treatment of peripheral arterial disease in diabetes written by the Italian Societies of Diabetes (SID, AMD), Radiology (SIRM) and Vascular Endovascular Surgery (SICVE). The consensus, published in Nutrition, Metabolism & Cardiovascular Disease (Aiello A et al, 2014; 24: 355–369), higlights that “the prevalence of peripheral arterial disease is high in diabetic patients and, associated or not with peripheral neuropathy, can be found in 50% of cases of diabetic foot.” Gargiulo said that the document summarises indications for revascularisation, revascularisation techniques and details on follow-up of revascularised patients, among other topics.
In the same session, Bijan Modarai spoke about effective cell therapies for revascularisation of critical limb ischaemia and Roberto Ferraresi discussed patient-centric revascularisation strategies.
At the end of the ilegx Collaboration Day a roundtable consensus on the role of drug-eluting balloons for the treatment of superficial femoral artery including data from the IN.PACT SFA, ILLUMINATE, Levant 2 and BIOLUX-PI studies was held. Vascular News will be reporting on the outcomes of this discussion.
Physicians share new product ideas at “Speed Dating” event
cxsymposium2015-07-11T02:23:23+01:00For the first time at the Charing Cross Symposium, physicians shared their product ideas with experienced physician-inventors, engineers and marketing experts in an event called “Speed Dating”. The event was held at the Innovation Showcase on Sunday 6 April. Physician-inventor David King (London, UK) won the “Dragons’ Den CX Innovation Showcase prize”.
Jean Bismuth, Elika Kashef and Stephen Greenhalgh, chairs of the session, designed the event to provide a platform for physicians who are seeking advice from experts on how to put their business and product ideas onto market.
Bismuth explained the rationale for the event: “Many young physicians and even senior physicians—as they gain experience through the years—have a desire to design their own medical tools and they start thinking and developing the idea. Unfortunately, sometimes they cannot get their innovations onto the market because they do not have the access to the experts to take the idea to the next level. With this “Speed Dating” event, we want to collaborate to bridge that gap.”
At the event, physicians had the opportunity to speak first to a group of physicians who have experience developing devices, then to engineers and finally to marketing experts. “Protect your idea” was the main advice that experts gave to the participants.
After the “Speed Dating” event, the Dragons’ Den CX Innovation session took place and awarded David King with £1,000 for his innovative idea. King designed a Doppler device called “Blue Dop” that works via a tablet device. He said that it uses a special algorithm that can measure blood pressure without the need to use a cuff or needles. He noted that the device is unique in the market and can be used in renal dialysis, arterial disease and sports medicine.
CX exposes alarming lack of physician awareness about radiation hazards
cxsymposium2016-10-12T13:01:21+01:00A session held yesterday at the Charing Cross International Symposium revealed that there is a widespread lack of physician awareness about the dangers of radiation in the interventional suite. The effect of exposure to radiation has become particularly important with the move from open to endovascular procedures, and delegates heard how no single specialty had a monopoly on bad practice when it came to radiation.
During the session it became apparent that the knowledge base about radiation had not been developed and incorporated into training and that appropriate behaviour in the interventional suite was often ignored, leading to operators receiving unnecessary doses of radiation. Surgeons, who have belatedly started using radiation, interventional cardiologists, interventional radiologists and radiologists are all guilty of bad practice, delegates heard.
“We are not aware of how badly affected we can be and this session turns the spotlight on how little we know. It is very important to become aware and realise the danger before you can start steps to reduce it. Perhaps as a profession we are not doing enough,” said Roger Greenhalgh, London, UK, chairman of the symposium.
Lindsay Machan, Vancouver, Canada, who was invited to comment on the Radiation Exposure session, told delegates that there were “two dirty little secrets” about radiation that he wanted delegates to pay attention to. “The first is that there is no safe dose of radiation; the idea that there is a threshold has now been debunked. The second is that every person in this room has a variant level of the radiation repair genes and there is no test as yet for the repair genes.”
Machan referred to the results of a long-term international study of thousands of workers exposed to radiation in the Chernobyl disaster in 1986, which found that the disaster clean-up crew, no matter where they were located, had the same incidence of cataracts, dispelling the idea of there being a threshold for cataract development. Referring to the outcomes from another 20-year study published by Chodick et al in the American Journal of Epidemiology in 2008 that found that there was no apparent threshold level for technologists to get cataracts, he said: “Everything that you hear about thresholds is absolutely not true. Treat radiation like iodinated contrast and use only as much as needed and no more.”
Machan also told delegates about the work of the late Basil V Worgul, New York, USA, which had shown that there was a possibility that the human population included genetically predisposed radiosensitive subsets.
“Everybody in this room has some degree of radiation damage and not everyone has complex innovations available to help them reduce the dosage. However, the distance from the tube and the importance of magnification cannot be overstated,” Machan said.
A member of the audience referred to currently available tools that enabled the visualisation of dosage in the form of a light or sound alarm. “Everybody wants to avoid radiation, they are just not aware of how their behaviour impacts dose. So if you stand unnecessarily close to the machine, there is an immediate warning and this can teach operators a lot,” he said.
Operator behaviour is driven by various factors
“You cannot overstate the importance of a real time reminder of the dose. Another important thing we have observed is that when our nurses and technologists became aware of their own risk, it changed the dynamic quite considerably. Operators are driven by a different dynamic; we want to get that procedure done, we want to show the photos and have a tendency to ignore [the radiation dose] and the consequences as they might be 20 years away. However, the nurses and technologists are not [driven by the same things]. They are there to do a job. As soon as they realise that they are at risk, it has an amazing impact. They start pointing out the fluoro time, question where they are asked to stand, and if you are doing a pedal puncture they might not stand behind the foot and hold the foot for you,” Machan said.
Another member of the audience made the point that they had measured and found that the radiation exposure was less in the hybrid suite as compared to their previous set-up. Their team had also found that the operator steps away just 6% of the time during digital subtraction angiography turns and have been working on educating the entire team. The awareness is lamentable, delegates heard.
Patients suffering burns
“One of the problems is that radiation burns can occur several months after the procedure and often the patient does not connect that to the procedure. Also, due to the fact that the image intensifier is above them, they think that the radiation dose is there. They do not realise why they have a burn on their back and often go to see a physician who does not have radiation in their mind as the cause, so there is a gross underreporting of the problem,” Machan said. The panel also commented that the biggest risks were for health professionals, as they are exposed repetitively during the course of their work. The maximum exposure resulted when branched devices were implanted, and the biggest risks are for health professionals as they are exposed every day.
Is regulation around the corner?
Machan made the point that while the health effects of radiation were one aspect, another issue was that of looming regulation for radiation workers. He pointed to the current practice in some US hospitals of radiation workers “sitting it out” if they had a high reading on their dosimeters. The sit-out period could range from anywhere between a week and a month. “Think of what this would do to your endovascular practice,” urged Machan. “If you have to sit out for a month every couple of months, you cannot actually practise.”
The panellists noted that while there was a wide variation in education and training requirements for personnel to be able to use radiation, there was need for special education for the whole team on to behave in an operating room. Machan also noted that while there were well laid out and appropriate guidelines in place, that the bigger problem was with adherence and enforcement.
Johannes Gahlen, Ludwigsburg, Germany, who spoke on the importance of radiation dosage exposure to patient and operator, said: “It is vital to adhere to the ALARA principle of keeping the dose as low as reasonably achievable. It is important to decrease the beam time, keep your distance from the system, use protective shielding, instruct the team, collimate the field of view, reduce the frame rate use use low dose programmes,” he said.
Following Gahlen’s presentation, Koning spoke about the novel X-ray system and how it can reduce radiation doses for patient and staff during endovascular procedures. He presented the results of a study and said that initial findings indicate that a significant radiation dose reduction of up to 75% to staff and patient can be realised for specific procedures.
He continued saying that radiation reduction in complex procedures, such as fenestrated stent graft implantation, means that implementation of lower doses of radiation is sometimes not attainable. He said that reducing radiation dose during the procedure will typically result in lower image quality, which may be unacceptable for clinical success of the endovascular treatment.
In the study, the investigators upgraded their existing C-arch to AlluraClarity (Philips Healthcare), which reportedly resulted in an ability to lower the required radiation exposure without losing image quality and without changing the workflow of the physician.
Koning reported that the results of the study are currently being processed for scientific publication. However, he said that initial findings indicate a significant dose reduction can be achieved. He said image quality was judged to be equal or superior compared to the image quality before installation and interventional work flows were uninterrupted.
Concluding, Koning said: “With the latest generation in imaging systems, we also see promise in increasing patient and staff safety without sacrificing the imaging quality required to provide optimal care.”
Still a lot to learn about abdominal compartment syndrome
cxsymposium2015-07-11T02:23:23+01:00At CX 2014, a mini symposium on abdominal compartment syndrome shed light on how little is known about the condition. It became apparent that the syndrome still has not been clearly defined, many of its triggering factors have yet to be recognised, and its management is limited to surgical decompression. Therefore, preventing both intra-abdominal hypertension and preventing intra-abdominal aortic hypertension from developing into abdominal aortic compartment syndrome is key.
Jan de Waele (Ghent, Belgium) stated that abdominal compartment syndrome has gone from being a syndrome that many vascular surgeons thought is “imaginary” to one that was now “being taken seriously.” He added that, according to the definitions of the World Society of the Abdominal Compartment Syndrome (WASCS), the syndrome was now defined as sustained intra-abdominal pressure of 20mmHg and it is associated with new organ dysfunction. “It can be a combination of haemodynamic problems, respiratory dysfunction, metabolic acidosis, and acute kidney dysfunction,” de Waele commented. However, he reported that “organ dysfunction sets in if you just look for it” at the threshold for intra-abdominal hypertension (a sustained pressure of 12mmHg or more). Therefore, he said it “made sense” to take steps to prevent both intra-abdominal hypertension and abdominal compartment syndrome. De Waele added: “We know the patients who are at risk, so we can really target intervention and we a have an indicator available—the intra-abdominal pressure.”
Anders Wanhainen (Uppsala, Sweden) explained that the WASCS had identified that risk factors for intra-abdominal hypertension and abdominal compartment syndrome included diminished abdominal wall compliance, increased intra-abdominal contents, capillary leak/fluid resuscitation, and increased intra-luminal contents. He noted that many of these risk factors were “relevant for vascular surgeons, particularly those managing patients with ruptured abdominal aortic aneurysms.” For example, Wanhainen explained, ruptured aortic aneurysms are associated with major trauma, intra-abdominal fluid collection, acidosis, hypothermia, polytransfusion, and shock or hypotension. He added: “Repair of a ruptured abdominal aortic aneurysm is definitely a high-risk procedure for the development of abdominal compartment syndrome.” According to Wanhainen, a study found that about 50% of patients undergoing open repair develop intra-abdominal hypertension and about 20% develop abdominal compartment syndrome. He added that these figures were, respectively, about 20% and 10% in patients undergoing endovascular aortic aneurysm repair (EVAR) but commented: “The observed lower risk after EVAR for a ruptured abdominal aortic aneurysm will probably change when more patients in shock are treated with EVAR.”
Wanhainen stated that because of the risk of intra-abdominal hypertension and abdominal compartment syndrome, intra-abdominal pressure “can and should be measured, preferably at the bedside in the intensive care unit” in patients undergoing emergency surgery or EVAR for a ruptured abdominal aortic aneurysm. He added that it was “completely possible” to eliminate the problem of abdominal compartment syndrome by taking adequate preventative measures—these measured included early bleeding control (which he called “crucial”), judicious fluid resuscitation, and prophylactic open abdomen management in selected patients. He added that preoperative and intraoperative factors could help to determine which patients should be selected for open abdomen management.
Martin Björck (Uppsala, Sweden) also stated that prevention was important in the management of intra-abdominal hypertension/abdominal compartment syndrome. In the context of EVAR/open repair, he said that prevention “started at the operating table.” He noted that this involved “a massive transfusion protocol and avoiding crystalloids,” agreeing with de Waele that it was “crucial” to monitor intra-abdominal pressure in all patients after aortic aneurysm repair in the postoperative period.
Björck noted: “We prevent abdominal compartment syndrome with aggressive medical management [in patients with intra-abdominal hypertension] but sometimes abdominal decompression is necessary.” He added, in terms of medical management, early pain relief could be “remarkably effective” and neuromuscular blockade, if the patient needs mechanical ventilation, was also very effective.
Björck reported that the recently updated 2013 WASCS guidelines were able to give strong recommendations for the management of abdominal compartment syndrome. He said that the guidelines recommend decompression laparotomy if abdominal compartment syndrome was present, protocolled effort should be made to obtain an early abdominal closure as “severe complications” can occur if open abdomen therapy is prolonged, and strategies using negative pressure wound therapy should be used. Björck agreed with de Waele that open abdomen treatment as a preventative treatment was an option as it “made sense” not to close a tense abdomen [in patients undergoing open repair] even though there are no data to support this approach.
However, he added if delegates used the preventative open abdomen therapy, they should close the abdomen “quickly”.
De Waele also spoke about the management of intra-abdominal hypertension/abdominal compartment syndrome, focusing on critical care. He said that the introduction of medical management options to decrease intra-abdominal pressure had “significantly changed the management of patients with intra-abdominal hypertension” and expanded on the WASCS recommendations—“The WASCS medical management algorithm identified five targets for medical interventions such as nasogastric decompression, neuromuscular blocking agents and percutaneous drainage among others. It is estimated that medical management can avoid surgery in a large proportion of patients with impending abdominal compartment syndrome.” However, de Waele said that surgical decompression remained an important element in the armamentarium and “may still be required” in some patients with therapy resistant abdominal compartment syndrome and significant organ derangement.” Concluding, he said that the management of abdominal compartment syndrome had now become the management of intra-abdominal hypertension as monitoring intra-aortic pressure was the “first and essential step” and prevention should be used where possible.
Charing Cross chairman Roger Greenhalgh (London, UK) told CX Daily News: “If I were a young physician, looking for an area to elucidate, I would choose abdominal compartment syndrome.”
No type I and III endoleaks with the Incraft system for EVAR at two years
cxsymposium2015-07-11T02:23:23+01:00Giovanni Pratesi (Florence, Italy) reported that the two-year results of the INNOVATION study (A multicentre, open-label, prospective, non-randomised study of the Cordis Incraft system in subjects with abdominal aortic aneurysms) confirm the “very promising results” of the one-year outcomes of the Incraft bifurcated stent graft system (Cordis). The results, presented at the Charing Cross Symposium 2014 (5-8 April, London, UK), showed that the system was not associated with any endoleaks at two years in patients undergoing endovascular aortic aneurysm repair (EVAR).
Pratesi stated that the Incraft system - showcased for the first time at the Charing Cross Symposium - has been designed to overcome the limitations of current stent grafts for the management of abdominal aortic aneurysms. He commented that it has an ultra-low profile delivery system and had the ability to be “customised during the procedure thanks to the bilateral in-situ length adjustment features”. Also, according to Pratesi, the graft offers the possibility of covering a wide range of anatomies with a minimum number of product codes.
The aim of the INNOVATION study was to assess the safety and efficacy of the Incraft system for the management of aortic abdominal aneurysms. The primary endpoint was technical success at one-month and the one-year safety endpoints included the absence of device- or procedure-related major adverse events, absence of type I or III endoleaks and maintenance of device integrity through one-year of follow-up. The main inclusion criteria included a proximal neck length of ≥15mm and up to 27mm in diameter, an access vessel large enough to accept the 14F outer diameter of the delivery catheter, and an aortic bifurcation >18mm in diameter.
The rate of technical success at one month was 97% (56 of 58 patients of the original 60 patients who were enrolled) and the rate of freedom from aneurysm enlargement was 100% at one year with the absence of both type I and III endoleaks in all patients.
Pratesti reported that two-year results confirmed these “very promising” early and one-year outcomes. He added that (at two years): “There were zero incidences of endoleaks (type I or type III), device- or procedure-related major adverse events, stent-graft migrations, or stent fractures.” He further said that there were also no incidences of aneurysm sac enlargement with sac shrinkage being observed in 45% of patients. However, one patient did develop a late limb ischaemia occlusion at day 666 due to sac contraction and limb confirmation change.
Pratesi concluded: “These excellent results are encouraging and suggest that the Cordis abdominal aortic aneurysm stent graft is a valuable alternative device with increased applicability over the broad spectrum of aorto-iliac anatomic configurations encountered in patients undergoing EVAR.”
CX Innovation Showcase highlights branched EVAR developments
cxsymposium2016-10-12T13:01:21+01:00Attendees at the CX Innovation Showcase learnt about the latest branched devices for endovascular aneurysm repair yesterday. New technologies for the vascular and endovascular treatment of lower limb, venous and carotid disease and arteriovenous access were also discussed. Stephen Greenhalgh (London, UK) and Andrew Holden (Auckland, New Zealand) were the chairmen of the session.
Greenhalgh told CX Daily News that the objective of this year’s session was “to get an idea of the pipeline of the major companies to tackle branched endovascular aneurysm repair.”
Among the branched devices featured at the CX Innovation Showcase, Michel Makaroun, Pittsburg, USA, presented on the investigational TAG thoracic branched endograft from Gore. He said that the design of this off-the-shelf device started “nearly a decade ago”.
The device has been built upon existing aortic and peripheral platforms and can be adapted to many anatomies. It is easy to use, requiring only femoral access and minimal manipulations, and is safe with minimal risk of branch coverage, he noted. The device has an aortic component which incorporates an internal portal allowing seal and fixation of the side branch component. The device diameters are between 21 and 53mm and the aortic treatment ranges between 16 and 48mm. “We have very early clinical experience with the device and it has been positive so far,” he concluded.
Zenith T-Branch and P-Branch endovascular grafts
Timothy Resch, Malmo, Sweden, spoke about Cook Medical’s Zenith T Branch and P Branch endovascular grafts, which are off-the-shelf systems for complex aortic repair. These devices, Resch noted, are aimed to “eliminate complex planning as well as manufacturing delays in the treatment of aortic aneurysms”.
The Zenith T Branch, designed to treat thoracoabdominal aortic aneurysms, has been CE-marked since 2012. Resch explained that the system consists of two main components that are compatible with the existing Zenith Thoracic Endovascular Aortic Repair (TEVAR) and iliac limbs components for proximal and distal extension depending on the aneurysm extent. The first component is a standard multi-branched device aimed at preserving flow into the main visceral side branches of the aorta. The second component is a “unibody” bifurcated device that comes in five different total lengths to match the individual anatomy. “Planning is simplified by using a predesigned planning sheet where the extent of the aneurysm as well as position of the target vessel ostia are marked,” he noted.
Resch highlighted that clinical and anatomical studies have determined that the applicability of the device in the treatment of thoracoabdominal aortic aneurysm ranges from 70 to 80% of the cases and multicentre studies are ongoing to evaluate further the applicability and efficacy of the device.
Resch went on to present the Zenith P Branch endovascular graft. He said that this device is designed to treat juxtarenal and suprarenal aneurysms as well as short neck infrarenal aneurysms which are not suitable for standard infrarenal EVAR. “As with the T Branch system, the P branch has two main components and is compatible distally with standard Zenith iliac limb extensions,” he said. In contrast to the T Branch, the P Branch it consists of a main body including two fenestrations for the renal artery and one for the superior mesenteric artery as well as a scallop fenestration for the coeliac trunk. Planning is done in a standardised fashion using a planning sheet and a template outlining the device.
Resch told delegates that anatomical studies in patients with juxtarenal abdominal aortic aneurysm have estimated the applicability of the P Branch to 50–70% but clinical data are still lacking. The device is not commercially available and international, multicentre feasibility studies are ongoing.
Relay branched endograft
In a subsequent presentation, Toru Kuratani, Osaka, Japan, talked about early and midterm results with the Relay branched endograft from Bolton Medical. He told delegates that he has been using the device, which is comprised of a flexible main body and two internal tunnels inside the main body, since 2013.
Early results in 12 patients treated with the Relay endograft have shown positive outcomes with 100% procedural success rate, no endoleaks, stroke or major complications, Kuratani noted. At six months and 12 months, there was a 100% survival rate and 100% freedom from aortic events. He said that endovascular repair with this device “may become a wonderful option for high risk patients.”
Martin Funovics (Vienna, Austria) presented a new branched endograft from Jotec. He told delegates: “If off-the-shelf technology fails, this is where the new technology from Jotec might jump in.” The device is adaptable to variable design choices and the branches are flexible with easy cannulation, he noted. He reported that the initial results were also promising.
The Nellix sealing system was featured at the Innovation showcase with presentations from Andrew Holden, Auckland, New Zealand, and Bob Mitchell, Hertogenbosch, The Netherlands. Mitchell told delegates that Nellix is a “game changer” in the treatment of abdominal aortic aneurysms. He said: “Nellix is not simply a next generation EVAR device, but it is rather a completely different approach to abdominal aortic aneurysm therapy. Nellix stands alone as the one and only alternative to EVAR.” This is a breakthrough concept designed to dramatically reduce known EVAR complications such as endoleaks, migration and reinterventions, he added.
As a highlight in the latest carotid innovations, Sumaira Macdonald, Sunnyvale, USA, spoke on the Michi system (Silk Road Medical), a cervical access approach that is used to reduce the risk of microemboli in carotid stenting. Macdonald told the audience that early results with the device “are encouraging” and “may represent a paradigm shift in the management of carotid artery disease.”
In the lower limb session, Michael Orlowski presented data for the Legflow Paclitaxel-Eluting Peripheral Balloon Dilatation catheter from Cardionovum.
David Deaton, Washington, USA, spoke on the Rox device for endovascular arteriovenous fistula; and Steve Elias, Englewood, USA, presented data on Clarivein, an endovenous ablation system used in the treatment of varicose veins.
Concluding the session, Greenhalgh told CX Daily News that there is a clear call from the audience for more evidence and more data from the technologies showcased. However, he added: “It is wonderful to see the rich pipeline of new devices that are coming.”
Physicians share new product ideas at “Speed Dating” event
For the first time at the Charing Cross Symposium, physicians shared their product ideas with experienced physician inventors, engineers and marketing experts in an event called “Speed Dating”. The event was held at the Innovation Showcase yesterday. Physician inventor David King (London, UK) won the “Dragons’ Den CX Innovation Showcase prize”.
Jean Bismuth, Elika Kashef and Stephen Greenhalgh, chairs of the session, designed the event to provide a platform for physicians who are seeking advice from experts on how to put their business and product ideas onto market.
Bismuth explained the rationale for the event: “Many young physicians and even senior physicians—as they gain experience through the years—have a desire to design their own medical tools and they start thinking and developing the idea. Unfortunately, sometimes they cannot get their innovations onto the market because they do not have the access to the experts to take the idea to the next level. With this “speed dating” event, we want to collaborate to bridge that gap.”
At the event, physicians had the opportunity to speak first to a group of physicians who have experience developing devices, then to engineers and finally to marketing experts.
“Protect your idea” was the main advice that experts gave to the participants.
After the “Speed Dating” event, the Dragons’ Den CX Innovation session took place and awarded David King with £1,000 for his innovative idea. King designed a Doppler device called “Blue Dop” that works via a tablet device. He said that it uses a special algorithm that can measure blood pressure without the need to use a cuff or needles. He noted that the device is unique in the market and can be used in renal dialysis, arterial disease and sports medicine.
IN.PACT SFA one-year results: Drug-eluting balloon outperforms angioplasty with lower reintervention rates and superior patency
cxsymposium2015-07-11T02:23:23+01:00The first-ever presentation of 12-month data from the randomised multicentre IN.PACT SFA trial revealed that treatment with the IN.PACT Admiral drug-eluting balloon (Medtronic) was significantly superior to percutaneous transluminal angioplasty in terms of reinterventions and patency. The results were presented at the Charing Cross Symposium 2014 (5–8 April 2014, London, UK).
The randomised controlled IN.PACT SFA trial results showed that clinically-driven target lesion revascularisation rates were significantly lower with the drug-eluting balloon as compared to those achieved with angioplasty (2.4% vs. 20.6%, p<0.001). Similarly, the primary patency rate achieved with IN.PACT Admiral was 82.2%, while the primary patency achieved with angioplasty was 52.45% (p<0.001). Primary patency at 360 days was also calculated by Kaplan-Meier survival estimates; at this specific time point, it was 89.8% for the drug-eluting balloon group and 66.8% for the angioplasty group.
According to Gunnar Tepe, Rosenheim, Germany, who presented the 12-month results of the trial, there is robust level 1 evidence to show that the IN.PACT Admiral drug-eluting balloon has achieved the lowest target lesion revascularisation and highest patency rates ever reported with an endovascular therapy in the superficial femoral artery.
An analysis of weighted average clinically-driven target lesion revascularisation rates at 12 months showed a lower reintervention rate with the Admiral drug-eluting balloon (2.4%) than with any other technology: 26.4% for percutaneous transluminal angioplasty, 14.3% for bare metal stents and 10.2% with drug-eluting stents.
“The results of this rigorously conducted randomised controlled trial warrant a review of current treatment guidelines for peripheral artery disease in the lower extremities,” said Tepe, who is the trial’s principal investigator. “In fact, they should lead to a reconsideration of how we treat patients with claudication, as the highest level of clinical evidence now distinguishes the IN.PACT Admiral drug-coated balloon as a primary therapy for atherosclerosis in the superficial femoral artery.”
The IN.PACT SFA trial enrolled 331 patients at 57 sites across Europe and the USA and patients were randomised to either receive treatment with a drug-eluting balloon or percutaneous transluminal angioplasty. To ensure data accuracy and reliability, patency endpoints underwent evaluation by an independent imaging core lab, while all clinical events were adjudicated by an independent clinical events committee. To prevent bias, both the imaging core lab and the clinical events committee were blinded to the patients’ randomisation.
The two cohorts were well-matched with regard to baseline patient demographics. Nearly all of the patients had moderate or severe claudication and approximately 5% suffered from rest pain because of more advanced disease. Other baseline characteristics including diabetes (40.5% vs. 48.6%) and hypertension (91.4% vs. 88.3%), as well as mean lesion length (8.94cm vs. 8.81cm) and percentage of total occlusions (25.8% vs. 19.5%) were also similar between the two groups.
The IN.PACT Admiral drug-coated balloon received the CE mark in 2009 but remains an investigational medical device in the United States, where it is under review by the FDA.
Audience recognises the impact of drug-eluting balloons
Following the presentation of the IN.PACT SFA data, the voting results at the Charing Cross Symposium 2014 captured a powerful shift in audience perception regarding the value of drug-eluting balloons in peripheral arterial disease. While just two years ago, a poll identified that 27% viewed drug-eluting balloons as an alternative to stents, in 2014, 72% now do so.
Voting questions from previous years were presented again this year, in order to assess the clinical views of audience members with regard to drug-eluting balloons. The results then captured the data on the year-on-year shift in perception towards these devices, and indeed the phenomenon of drug elution itself. The audience response polls revealed that nearly 90% voted that drug-elution was worthwhile in the superficial femoral artery. Last year, just 57% cast their vote stating that drug-elution was worthwhile in this anatomical region.
Roger Greenhalgh, London, UK and chairman of the symposium, noted that the voting trends likely reflected the multidisciplinary nature of the meeting itself as the audience members and faculty are composed of the leading vascular surgeons, interventional radiologists and interventional cardiologists.
Two years ago, when delegates at Charing Cross 2012 were asked what the role for drug-eluting balloons was, 27% voted that they were an alternative to stents; 33% voted that they should be used for in-stent restenosis; 19% voted that they should be used as a last resort and 21% voted that they had no role. Now in 2014, 72% voted that drug-eluting balloons are an alternative to stents; 21% voted that they should be used for in-stent restenosis; 3% believed that they should be used as a last resort and 4% voted that they had no role at all.
There was a similar swing in opinion regarding the cost-effectiveness of drug-eluting balloons. In 2011, 75% of voters stated that they did not find drug elution cost-effective in the superficial femoral artery and only 25% voted that they did. Yet in 2014, 67% said, yes, drug elution is cost-effective in the superficial femoral artery and 33% said it was not.
The voting also revealed a sharp shift seen in favour of drug-eluting balloons from last year. In 2013, 57% of the delegates voted that drug elution was worthwhile in the superficial femoral artery, while 43% believed that it was not. In contrast, in 2014, an overwhelming 87% agreed with the proposition “drug elution is worthwhile in the superficial femoral artery” while 13% did not.
Leave nothing behind: Bioresorbable devices are an “exciting prospect” for the future
cxsymposium2016-10-12T13:01:21+01:00Andrew Holden, Auckland, New Zealand, presented data for on the developments in bioresorbable technology yesterday. He said that, as drug-eluting balloon data are limited to short and intermediate length lesions, bioresorbable stents or scaffolds are an “exciting prospect” for the future.
He said that the long and mobile femoropopliteal arterial segment is a challenging environment for endovascular intervention; for more complex lesions a scaffold is often required to prevent residual stenosis and flow-limiting dissection. An anti-restenosis strategy is also important, particularly in claudicants where long-term patency is vital, he noted.
“Drug-eluting balloons are highly promising but have only been studied for any duration in relatively short lesions. Drug-eluting stents have shown satisfactory patencies in intermediate length lesions but suffer the problems of a permanent self-expanding metallic implant.”
Addressing this problem with drug-eluting stents, Holden said for many years bioresorbable stents have been eagerly awaited in the superficial femoral artery.
“A bioresorbable stent may provide a scaffold to optimise the acute result after angioplasty without the long-term irritation of a self-expanding stent. Such a scaffold must withstand the hostile environment of the superficial femoral artery, provide mechanical support and integrity through vessel healing, remain biocompatible through resorption and facilitate drug delivery,” he said.
Three bioresorbable stents have recently been studied in the superficial femoral artery. The Abbott Esprit 1 trial used a balloon expandable PLLA scaffold in iliac and femoral arterial lesions ≤50mm in length that could be treated by a single 6.0mmx58mm device. The study reported excellent procedural success. Although lesion length was short (mean 35.7mm), the patency data and improvement in Rutherford-Becker status at one year was very encouraging, Holden said.
The 480 Biomedical Stanza stent has been studied in the STANCE trial. This flexible, self-expanding stent is a PLGA and bioreorbable elastomer composite and fully resorbs in 12–15 months, according to Holden. Acute performance and subsequent stent strut encapsulation and resorption has been evaluated with optical coherence tomography (OCT). This study reported excellent procedural success and acute stent performance, treating longer lesions (up to 90mm).
Holden explained that late lumen loss seen in the first cohort of patients was due to a combination of vessel recoil and neointimal hyperplasia. The device was modified in a second patient cohort with minimal vessel recoil. A paclitaxel, drug-eluting version of the scaffold has recently entered the clinic in the SPRINT trial, he added.
Holden also reported that the Igaki-Tamai bioresorbable scaffold (Igaki Medical Planning) has been used in the superficial femoral artery in a 30 patient cohort. Acute procedural results were very good, as in the STANCE trial.
“Binary restenosis rates at 12 months were unacceptably high and further modifications are planned. Small clinician initiated trials using coronary bioresorbable stents in the tibial arteries are being performed but meaningful results are not yet available,” Holden commented.
“There has been considerable progress with bioresorbable stents in the superficial femoral artery. The technology is not yet ready for routine clinical practice but that exciting prospect should not be far away,” Holden concluded.
“New approach to superficial femoral artery reconstruction is highly versatile”
cxsymposium2016-10-12T13:01:21+01:00In his technical report of the Hybrid Vascular Graft (Gore) for the reconstruction of the superficial femoral artery, Jean Bismuth (Houston, USA) said the graft allowed a rapid sutureless anastomosis and was highly versatile in complex situations.
Bismuth reported that the graft is the first device that has been designed to “address the ever expanding number of hybrid operations, which combine both endovascular and open surgical techniques.” He explained that it was an expanded PTFE vascular prosthesis that had a constrained nitinol section and added that it “greatly expanded” the treatment options for dialysis access, aortic debranching, and arterial bypass procedures. Bismuth commented: “The graft allows the surgeon to minimise the invasiveness of a procedure all the while benefiting from the advantages of a bypass.”
He explained that although initially the graft was launched for arteriovenous dialysis access, it now had the same indications as “any vascular graft”. For example, at his centre, where the graft has been used in 150 cases, it has been used for “a good proportion of iliofemoral bypasses and a bunch of visceral debranching as well.”
Bismuth commented: “In the femoropopliteal segment, the Hybrid graft permits access through a smaller incision to an artery which is either hard to reach behind the knee or is diseased. The nitinol reinforced segment of the graft can be introduced through a less invasive access and can take advantage of the stent to treat a stenotic segment.”
As well as reviewing the advantages of the graft, Bismuth also discussed the “pearls for success” with the device and these included the fact that “imaging was key” to successfully accessing patient anatomy—“you have to know what you are going into”, he noted. Another pearl was that correct sizing was “essential” as he said that the graft should not be oversized by more than 20%. Bismuth added that he never oversized by more than 1mm and that correct sizing could mean that sutures were not needed at all. He explained: “Gore recommends a couple of patching sutures, but I have never put any in. It is a personal choice and I have never had an issue with needing to use sutures. It is all about the sizing. If you only upsize by 1mm, you are probably not going to have any problems.” Other tips for success included not completely inserting the nitinol reinforced segment into the artery, ensuring adequate outflow and inflow, and covering all of the disease in the artery.
Bismuth concluded: “Hybrid procedures are likely to be more prevalent; the Hybrid graft allows a rapid sutureless anastomosis, particularly for difficult sites and rapid bailout. It is highly versatile in complex cases.”
BASIL 2 randomised trial launched to address need for data for endovascular interventions for severe limb ischaemia
cxsymposium2015-07-11T02:23:23+01:00Andrew Bradbury (Birmingham, UK) reported that a new randomised controlled trial, BASIL 2, has been launched because there is an “urgent need” to undertake pragmatic, scientifically robust and publically-funded randomised controlled trials of endovascular interventions in patients with severe limb ischaemia.
Bradbury stated that the findings of BASIL 1, which showed a significant improvement in overall survival with endovascular therapy in patients with severe limb ischaemia compared with surgery at 7.3 months (but not at two years), and those of other studies have been used to justify “an endovascular approach” in this group of patients. However, he added that BASIL 1 may no longer be relevant because of several changes in the treatment of severe limb ischaemia since the study was published. For example, Bradbury reported, endovascular therapy had “changed beyond recognition”, interventional radiologists were more skilled at performing endovascular therapy, and surgeons could now perform hybrid procedures. Furthermore, other studies in this area were industry sponsored and had not produced the answers needed to make nation-wide decisions about which therapy to use. Bradbury said, during development of guidelines for peripheral artery disease, the UK’s National Institute for Health and Care Excellence (NICE) were “shocked” that the day-to-day decisions for the management of severe limb ischaemia were being based on such a “lack of evidence”.
Therefore, the BASIL 2 has been launched to provide more data on the effects of endovascular therapy compared with surgery in patients with severe limb ischaemia. Bradbury reported that in the superiority trial, 600 patients with below-the-knee or femoropopliteal atherosclerosis will be randomised to receive “best endovascular therapy” or a vein graft. He added that the recruitment process, which was due to start in a few weeks, would last for 36 months and the primary endpoint was the rate of amputation-free survival at 33 months. To show superiority of endovascular treatment, Bradbury reported, there needs to be a 15% difference in the primary endpoint between the groups.
The study will be funded by the National Institute for Health Research and, according to Bradbury, has received “overwhelming support” from both interventional radiologists and vascular surgeons. He acknowledged that recruitment for BASIL 1 had been difficult—“it is the reason why my hair is grey; in fact, it is a wonder that I have any hair at all”—but said the vascular community were now more accepting of the need for randomised controlled trials.
Concluding, Bradbury said that there was an “urgent need” to undertake pragmatic, scientifically robust and publically-funded randomised controlled trial of endovascular therapies in severe limb ischaemia that were powered for clinically important endpoints and include a full cost-effectiveness analysis.” He added that the BASIL 2 would look at the health costs and, for “the first time”, the social costs of the interventions in the study. “Without data from such randomised controlled trials, we cannot be sure that endovascular interventions are not going to be associated with net harm or suboptimal use of precious health resources,” Bradbury said.
Practical issues arising from hyper-acute carotid interventions
cxsymposium2015-07-11T02:23:23+01:00In Leicester, UK, rapid-access surgery for symptomatic carotid stenosis has been offered since October 2008. Ross Naylor reviews the practical lessons that have been learned with the experience. He discussed this topic at CX35 on Tuesday.
By Ross Naylor
When the trials randomised “recently symptomatic” patients to carotid endarterectomy or medical therapy, symptoms had to have occurred
Consequently, there has been a move towards performing surgery as soon as possible after the index event (ie treating transient ischaemic attack on a par with unstable angina). NICE advise that patients should undergo surgery
Some surgical/interventional colleagues have not actively embraced the move towards expedited intervention. For them, patients benefit from a period of stabilisation and assessment (in order to reduce procedural risk), while others believe that intervening early is associated with an unacceptably high procedural risk that may negate any benefit conferred through early intervention. The reality is, however, that the surgeon who operates within two weeks with a 10% procedural risk is still likely to prevent more strokes (in the long term) than the surgeon who defers surgery for four weeks and then operates with a 0% risk!
The Leicester Unit has offered a rapid-access surgery service since October 2008. All patients are seen in a 24/7 cerebrovascular clinic and those with 50–99% stenoses are transferred to the Vascular Unit for expedited surgery. 400+ symptomatic patients have now been treated (12%
First; it is not unusual (in the hyper-acute setting) for the duplex operator to comment that there is a critical stenosis that does not appear to open out into a normal calibre vessel. In the past (when patients were randomised some time after the index event), this might have been diagnosed as “near occlusion” (little benefit from surgery), but this is not the case in the hyper-acute setting. The diagnostic “give-away” is that high velocities are maintained across the stenosis (even if the distal lumen cannot be visualised) and CT angiography almost always shows a reconstructable vessel. In “near occlusion”, there are very low systolic velicities and little or no diastolic flow. Accordingly, corroborative imaging is mandatory (in the hyper-acute period) before recommending against urgent carotid surgery.
Second; anyone setting up this kind of service better get used to seeing recurrent events in up to 15% of patients between admission and surgery (despite being on antiplatelet and statin therapy). Up to 40% of patients referred acutely will have spontaneous embolisation on transcranial Doppler. Recurrent events (prior to surgery) were rarely encountered in the past, largely because patients were not referred so quickly.
Third; patients undergoing surgery (using general anaesthetic) with a pre-existing neurological deficit will almost always suffer a transient worsening of their deficit postoperatively. In the past, this was an indication for re-exploration. The “key” to management is how quickly the patient recovers from anaesthesia. If it is relatively quick, they will return to their pre-operative neurological status within an hour or so. Accordingly, it is important to warn recovery staff who may otherwise be alarmed at the apparent neurological deterioration.
Fourth; be prepared to adopt even more obsessive attention to surgical technique. We have found that careless skin preparation can trigger embolisation and transcranial Doppler is invaluable in warning of the embolising (unstable) patient during carotid mobilisation.
Fifth; be prepared to encounter a higher prevalence of post-endarterectomy hypertension (25% in theatre recovery, 25% back on the ward). This is usually seen in patients with poorly controlled blood pressure pre-operatively and it is essential that medical/nursing staff have guidelines for managing this condition. If you are going to treat patients in the hyper-acute period, you cannot adopt an ad hoc approach to blood pressure management, as patients will be subject to a greater risk of hyperperfusion stroke or intracranial haemorrhage.
Finally; (and contrary to what has previously been expected), our experience of operating in the hyperacute period has not been associated with a significant increase in procedural risk.
However; if you still harbour doubts about the benefit of intervening in the hyperacute period, ask yourself how you would want to be treated should you suffer a transient ischaemic attack and have a significant carotid stenosis?
Thought so! Don’t your patients deserve the same?
Diffusion-weight magnetic resonance imaging in carotid artery interventions
cxsymposium2016-10-12T13:01:23+01:00Following carotid intervention, the number of detectable diffusion-weight magnetic resonance imaging (DW-MRI) lesions is an order of magnitude greater than adverse clinical event (stroke/death). This lends credence to the use of DW-MRI as a surrogate endpoint allowing comparisons of interventional strategies in studies with reduced sample size, wrote Sumaira Macdonald, Newcastle, UK. She discussed this topic at CX35 on Tuesday 9 April.
By Sumaira Macdonald
The International Carotid Stenting Study (ICSS) sub-study comparing DW-MRI lesions in patients undergoing largely filter-protected carotid stenting and carotid endarterectomy demonstrated significantly fewer DW-MRI lesions after carotid endarterectomy, implying superior of control of procedural microemboli. Sixty-two of 124 (50%) patients undergoing distal filter-protected transfemoral carotid artery stenting and 18 of 107 patients undergoing carotid endarterectomy (17%) had new DW-MRI lesions (p<0.0001). Individual lesions were smaller in the carotid artery stenting group than in the carotid endarterectomy group (p<0.0001). Of the DW-MRI positive scans following carotid artery stenting, 25 (34%) resulted from unprotected carotid artery stenting and 37 (75%) resulted from filter-protected carotid artery stenting (p<0.019). Total lesion volume per patient did not differ significantly between patients undergoing carotid artery stenting and those undergoing carotid endarterectomy.
Two small randomised trials compared proximal embolic protection (Medtronic MoMa) with distal filters during carotid artery stenting. There were substantial or significant reductions in DW-MRI lesions for the MoMa compared with filter protection.
There were significantly fewer DW-MRI lesions in the MoMa group ipsilateral to the carotid lesion (p<0.0002) but no difference in the DW-MRI lesions in the contralateral hemisphere, implying the embolic penalty associated with catheterisation of the arch/great vessel origins for transfemoral carotid artery stenting. There was also a significant difference in favour of the MoMa system for lesion volume 0 [0 to 0.84] vs. 0.47 [0 to 2.4cm3] (p<0.0001).
The PROOF first-in-man analysis of high flow rate flow reversal via direct common carotid artery access (MICHI System) evaluated 65 patients, 48 of who had pre- and post-carotid artery stenting DW-MRI read by two independent US neuroradiologists. Eight of 48 patients had new DW-MRI lesions (16.7%).
Another study examined patients undergoing transcervical carotid artery stenting with flow reversal or distal filter-protected transfemoral carotid artery stenting. DW-MRI lesions were found in four of 64 transcervical (12.9%) and in 11 transfemoral (33.3%) patients (p=0.03). In multivariate analysis, age (relative risk, 1.022; p<.001), symptomatic status (relative risk, 4.109; p<.001), and open-cell vs. closed-cell stent design (relative risk, 2.01; p<.001) were associated with a higher risk of lesions in the transfemoral group but not in the transcervical group.
The low rates of DW-MRI lesions in studies of carotid artery stenting with flow reversal via direct carotid access are commensurate with carotid endarterectomy, presumably resulting from more effective embolic control and avoidance of catheterisation of the arch.
A prospective study of 110 patients undergoing filter-protected transfemoral carotid artery stenting investigated the fate of silent DW-MRI lesions. Twelve of 30 DWI lesions persisted, resulting in a lesion reversibility rate of 60%. Seventy-five per cent (12/16) of the cortical lesions disappeared while only 30% (3/10) of subcortical lesions disappeared. Eighty-three per cent (14/17) of lesions measuring 0–5mm disappeared while only 31% (4/13) of lesions measuring >5mm disappeared. It was concluded that a large number of silent ischaemic lesions visualised on the DWI images post-carotid artery stenting disappear within months and therefore the extent of permanent carotid artery stenting-related cerebral damage may be overestimated.
The most recent analyses of the ICSS sub-study data set revealed that patients in the carotid artery stenting group had more acute (relative risk 8.8, 95% CI 4.4-17.5, p<0.001) and persisting lesions (relative risk 4.2, 1.6-11.1; p=0.005) than patients in the carotid endarterectomy group. However, the rate of conversion from acute to persisting lesions was lower in the carotid artery stenting group than in the carotid endarterectomy group (RR 0.4, 0.2-0.8; p=0.007).
Systematic reviews have failed to provide consistent data across included studies comparing cognitive outcomes following carotid artery stenting and carotid endarterectomy.
Of 1,713 patients included in the ICSS, 140 of 177 patients enrolled in two Dutch centres had neuropsychometric testing at baseline and 120 at follow-up. Ten domains were examined, including executive function. There were no significant difference in overall cognition between patients undergoing carotid artery stenting and carotid endarterectomy despite the impressive difference in DW-MRI lesions counts between carotid artery stenting and carotid endarterectomy.
“Standard” filter-protected transfemoral carotid artery stenting generates more DW-MRI lesions than carotid endarterectomy but technical modifications (proximal embolic protection, direct carotid access) allow carotid artery stenting to more effectively compete where microemboli are concerned. Clinical correlation, with regards cognitive function is poor, implying either that a large number of DWI lesions are clinically irrelevant or that neuropsychometry is a blunt tool. DW-MRI is a reasonable secondary endpoint for carotid interventions, but without watertight clinical inference, the use of DW-MRI as a primary endpoint remains an unproven convenience.
CX Non-Cardiovascular Imaging Day embraces other specialties
cxsymposium2016-10-12T13:01:23+01:00On Tuesday 9 April, in the CX Non-Cardiovascular Adavanced Imaging Day session, Roger Greenhalgh, London, UK, introduced the speakers from the respective companies who spoke about building a hybrid operating room, the financial implications of doing so, and laser-guided hybrid suites.
In the afternoon session, chaired by John Primrose, president of the association of Surgeons of Great Britain and Ireland, showed delegates on the following topics:
- Florian Gebhard, Ulm, Germany—Making the hybrid operating room cost effective: the multidisciplinary hybrid operating room, and improving trauma care
- Manuel Ritter, Mannheirn, Germany— Radiation safety: management of dose in the hybrid operating room, and improvement of PCNL by Uro-Dyna computed tomography (CT)
- Helmut Isringhaus, Völkinghaus, Germany—Minimally-invasive resection of small lung nodules guided by fluoroscopy
- Javier Fandino, Aarau, Switzerland—Concept and applications of the hybrid operating room in cerebrovascular surgery
- Dogu Teber, Heidelberg, Germany—Laparoscopic partial nephrectomy in kidney cancer guided by Dyna CT/laparoscopy image diffusion
- Marco van Strijen, Nieuwegien, The Netherlands,—Advanced image guidance in renal tumour ablations
- Beat Müller, Heidelberg, Germany—Image-guided partial liver resection in hepatocellular carcinoma
- Alexander Schramm, Ulm, Germany—First experience of maxillofacial surgery in a hybrid operating room
- Chrisitan Raftopoulos, Brussels, Belgium—The hybrid operating room for neurosurgery
- Hicham Kobeiter, Créteil, France—Advanced image guidance for transcatheter arterial tumour embolisation using EmboGuide
CT is the best imaging modality for deep vein thrombosis
cxsymposium2016-10-12T13:01:23+01:00Gerard O’Sullivan, Galway, Ireland, spoke in the Venous Challenges sessions on Tuesday 9 April about imaging for iliac deep vein thrombosis and the best modalities to use. He told delegates that computed tomography pulmonary angiography (CTPA) and CT venography are his preferred modalities.
I do a lot of acute iliofemoral deep venous work, and I do not think there is one modality that covers it all. If I had lots of MRI scanners and a lot of time I would use MRI much more, but I do not. When you are doing iliofemoral deep venous work for thrombosis, what kills the patient is pulmonary embolism and right ventricular dilatation. In my opinion, you need to evaluate the right ventricle and the pulmonary arteries when you are evaluating this patient. You can do that by a variety of techniques, but for me CTPA is the quickest,” he said.
Comparing CTPA and CT venography vs. MR venography, O’Sullivan noted that CT is quicker but involves the use of radiation. “It is less elegant but you can see more the inside of vessels,” he stated. “We looked at our data and, despite previous works suggesting that one-third of patients had positive CTPA with deep vein thrombosis, we found that 79% of our patients had had a pulmonary embolism by the time they presented for iliofemoral deep venous acute treatment.”
He explained that what physicians should be looking for is not pulmonary embolism, but right ventricular dilatation. O’Sullivan said that the pulmonary arteries should be assessed in several ways: isotope plus direct venography, isotope or CTPA plus ultrasound of the legs, CTPA plus CT venography, MR pulmonary angiogram plus peripheral MR venography, or echocardiography plus peripheral MR venography.
“In practical terms, for me CTPA plus CT venography is the better method as I can do it quickly and have all the information I need. I also use ultrasound to assess the popliteal vein as the further down you go more difficult it is to interpret CTPA,” he said.
He explained that in Galway he performs indirect CV venography with standard peripheral intravenous injects at same sitting at CTPA, 20G cannula in wrist or elbow, 150cc iodinated contrast, image at 150s, and 5mm cuts from diaphragm to mid-calf. “The pictures are not comparable to CT angiography but with experience are easily adequate for diagnosis.”
O’Sullivan concluded by saying that CT venography and MR venography are essential in addition to ultrasound in preoperative assessment of iliac venous thrombosis. “We feel limited ultrasound with CTPA and indirect CT venography offers the most rapid diagnostic combination method to assess pulmonary embolus, right ventricular dilatation, inferior vena cava thrombus, acute vs. chronic disease above and below the groin and extra-vascular lesions eg tumour. But other physicians use other methods equally well or better.”
Renal denervation should not be part of routine clinical practice, delegates say
cxsymposium2016-10-12T13:01:23+01:00At today’s CX Renal Denervation Session Great Debate, delegates voted 3:1 against the motion that renal denervation should be used routinely to treat hypertension. As all of the speakers, regardless of which side of the debate they were, agreed that renal denervation should only be used to treat patients with resistant hypertension, the real focus of the debate centred on what was meant by the word “routine”.
Mel Lobo (London, UK), who was supporting the motion that renal denervation should be part of routine clinical practice to treat hypertension, claimed that the definition of routine was a “key issue” in the debate. He explained that patients who were suitable candidates for the intervention belonged to a highly selective group of patients, who had true resistant hypertension (ie. non-concordance with medication and other causes of resistant hypertension had been ruled out) and who would be managed by hypertension specialists rather than by primary care physicians. Lobo added that, in his view, renal denervation should become “step five” of the UK National Institute for Health and Clinical Excellence (NICE) pathway for the management of hypertension, commenting: “We do not have any randomised controlled trial data for step four [the use of spironolactone; only observational data are available], so why not have renal denervation as step five? Believe me when I say adding in a sixth or seventh antihypertensive drug is not going to work.”
Felix Mahfoud (Homburg, Germany), who was Lobo’s co-proponent of the motion, agreed that the routine use of renal denervation referred to it being used only in the “very specialised” group of patients with true resistant hypertension. He said: “Is it really ethical not to offer patients a modality that could benefit them?”
Speaking against the motion, Mark Caulfield (London, UK) disputed that “routine” could refer to an intervention being used in a highly selected group of patients. Caulfield commented: “Routine use means everybody; it implies that the intervention should probably be used in a large number of patients. Renal denervation is not ready to be used in routine clinical practice because there is not evidence base to support it being used in this way.” He added that the NICE consensus document on renal denervation, of which he was a co-author, was specifically drawn up because there were concerns that the doctors would use the intervention for all patients with hypertension rather than just those with true resistant hypertension.
Also speaking against the motion was Bryan Williams (London, UK), who said he was doubtful about the data from renal denervation studies. He commented: “What is critical is how many patients in these studies were actually taking their antihypertensive medication? If the drop in blood pressure observed in these studies is really a result of renal denervation on top of antihypertensive medication, then that is impressive. But if it is actually a result of patients starting to take their drugs [because they are now in a clinical trial], then it is not impressive.”
Lobo responded by saying there had to be element of trust and that “We have to believe our patients when they say they take their medication. We are not able to follow them home and check up on them.”
At the end of the debate, 3:1 delegates voted to support Caulfield and Williams that renal denervation should not be part of routine clinical practice for managing hypertension. Summing up the data, the chair of the session Neil Poulter (London, UK) said he hoped that by the time of CX36 (12–15 April 2014), more data on renal denervation would be available and the issue could be further discussed.
Endovascular management of ascending aortic pathology
cxsymposium2016-10-12T13:01:23+01:00By Ralf Kolvenbach
The incidence of thoracic aortic aneurysms is estimated to be as high as six cases per 100,000 person-years, and replacement of the ascending aorta accounts for the majority of cardiothoracic aortic procedures.
Aneurysms and dissections of the ascending aorta are still mainly treated operatively with cardiopulmonary bypass. Ascending aortic aneurysms with normal sinuses and aortic annulus require only replacement of the ascending aorta from the sinotubular ridge to the origin of the innominate artery with a Dacron tube graft.
Due to the significant morbidity and mortality of these procedures high-risk patients are not considered for cardiac surgery. There are a number of anecdotal reports describing endovascular stent grafting of various pathologies including deployment of a fenestrated stent graft in a case of an ascending aortic rupture.
Endovascular treatment of the ascending aorta is particularly challenging because of the anatomical features of this aortic segment. Acute and chronic Type A dissections can be treated with a tubular endograft when the aortic valve and the coronary arteries are not involved. An analysis in our clinic of 30 consecutive patients admitted with ascending aortic pathology showed that in 75% ascending aneurysms had a conical shape without a proximal landing zone. Only in a few cases the ascending aorta had a tubular configuration with a long proximal landing zone of at least 2cm. This makes over-sizing and an optimal graft configuration particularly important. Only patients without connective tissue disorders, clinically relevant aortic incompetence, stenosis or concomitant coronary artery disease can be considered for an endovascular procedure.
Material and methods
So far only patients with ascending aortic pathology who were considered unfit for open surgery were treated with an endograft. We also excluded patients with malperfusion or any preoperative unstable clinical condition after Type A dissection. Also excluded were patients with severe aortic valvular disease including aortic valvular incompetence as a consequence of an acute Type A dissection, coronary artery disease requiring surgery, and any kind of connective tissue disorder. Included in our ongoing study are patients with intramural hematoma, floating thrombus after chronic Type A dissection and penetrating aortic ulcers. Also included are aneurysm patients without aortic valve incompetence or significant dilatation of the aortic annulus requiring composite graft replacement of the aortic valve and the ascending aorta.
Patients with asymptomatic penetrating aortic ulcers (PAU) were initially managed conservatively. If symptoms did not resolve, or patients continued complaining about chest pain, endovascular exclusion was discussed with the patient.
Threshold for aneurysm patients is an aortic diameter of 6cm or larger and an adequate landing zone proximal to the coronary arteries. Patients were excluded if they were good candidates for open surgery. In cases without a good landing zone an aortic banding procedure using a mini sternotomy was performed prior to stent graft deployment as originally described by the Zurich group of Lachat.
The length of the stent graft was depending on the length of the outer curve of the ascending aorta which was regularly longer than the distance determined by center line measurement.
All operations were performed under general anesthesia. In addition to our monitoring protocol for TEVAR transoesophageal ultrasound was performed to control cardiac and valvular function. Cardiac arrest was induced with adenosine administration when required. A temporary ventricular pacemaker was placed through the jugular vein.
After road map angiography selective coronary angiography was performed to outline the origin of the coronary arteries.
An ultra stiff guide wire (Lunderquist, Cook) was placed into the left ventricle after passage of the aortic valve with a vertebral catheter over a 0.035 guide wire. The endograft (Cook custom made ascending endograft) was carefully placed across the aortic valve into the left ventricle. The kind of graft used depended on the size required.
Tracking of the stiff wire all the time during the procedure was essential to avoid ventricular perforation. At the end of the procedure ventriculography was performed to rule out any damage to the left ventricle or the valve apparatus. In case of any ECG changes coronary angiography was added before removing the catheter and sheaths. Before discharge a contrast enhanced angio CT was performed as well as cardiac ultrasound examination.
Ascending aortic dilatation may be caused by intrinsic pathology of the aortic wall, or hemodynamic factors caused by a stenotic aortic valve: High velocity and turbulent flow downstream of the stenosis place mechanical stress on the aortic wall.
The thin wall of the ascending aorta does not permit aggressive over dilatation. The graft was deployed in most cases without any additional balloon dilatation. The walls of the aortic sinus (sinus of Valsalva) are considerably thinner than the wall of the aorta. Hooks or bear springs should therefore be deployed in a safe distance from the sinus and the origin of the coronary arteries to avoid erosion and perforation. One of our first patients suffered from a stroke postoperatively.
Calcifications and thrombus lining of the ascending aorta and aortic arch were the main parameters for increased stroke risk
Access can be a problem since in most aneurysm cases a 24F access sheath was required. In two cases with sever calcification of the iliac vessels we had to use the left carotid artery as an access which proved to be uneventful. Alternatively using a small thoracotomy the endograft can be deployed through the apex of the heart.
There is so far only one dedicated graft for the ascending aorta (Cook). Since most aneurysms have conical shape a stent graft designed for Type a dissection cases is not necessarily suitable for aneurysm patients. So far the anchoring zone of a few millimeters only, requires fixation with hooks, significant over sizing and coil deployment into the sac of the aneurysm if necessary. Banding of the ascending aorta is an option to increase the landing zone in aneurysm patients with conical aneurysm morphology.
The aortic impulse which is directly proportional to the mean blood pressure, the cross sectional area of the aorta and the duration during systole varies inversely with the distance from the aortic valve. In stent grafting of the aortic arch and the descending aorta high aortic impulses can cause significant pulsate motion of the arch and inadvertent movement of the stent graft. In the future more active fixation with a stapler would probably permit safer deployment, less Type I leaks and better long term performance. Especially when treating more advanced stages of aortic pathology a valve bearing conduit with fenestrations for the coronary arteries will be necessary.
Before a routine use can be advocated outside of studies several issues must be addressed. There should be dedicated grafts for ascending aneurysms and for acute dissections like the one available so far. Precise deployment of the graft adjacent to the coronary arteries is essential in aneurysm patients. In most cases there is a landing zone of a few millimeters only, deployment too distal from the sinotubular junction results in kinking of the graft and subsequently a type I leak.
We can conclude that stent grafting of the ascending aorta is technically feasible but should be reserved to selected high risk patients only, preferably in centers where vascular specialists cooperate closely with interventional cardiologists. Cardiac surgery with cardiopulmonary bypass and if necessary deep hypothermia is still the gold standard when treating ascending aortic aneurysms though still associated with significant morbidity and mortality. Stent graft exclusion of more advanced and complex ascending aortic aneurysms should be reserved for high risk patients only in centers with the necessary skills to perform transvalvular cardiac procedures. This may change in the future when more dedicated grafts become available. The future will and has to show valve bearing endovascular conduits which will permit minimal invasive treatment of most aortic aneurysms. The grafts available so far already permit emergency endovascular treatment of type A dissections. Yet in aneurysm patients compromises are still necessary.
Clinical need drives intraoperative imaging to the next level
cxsymposium2016-10-12T13:01:23+01:00For the very first time, three full-scale sterile hybrid operating suites from GE Healthcare, Philips and Siemens were on display at CX. The importance of quality imaging as a prerequisite for improved clinical outcomes was emphasised in every section of the main programme. This went hand in hand with calls from physicians for high-quality image availability in the intraoperative setting.
Imaging is certainly at the heart of endovascular intervention, and it is now widely accepted that using the best available imaging can have a direct impact on achieving the best clinical results. With the blurring of boundaries between specialties in the endovascular arena, there was a clear need expressed by clinicians at CX for improved imaging at the intraoperative stage. Delegates at CX35 are split in nearly equal proportions along the disciplines of vascular surgery, interventional radiology and interventional cardiology.
Georg Nollert, director, Global Marketing, Siemens, told CX Daily News: “I am very happy that the focus on imaging is increasing. In the past, vascular surgeons were satisfied with inferior image quality and other interventionalists such as radiologists and cardiologists benefited from using the best available imaging. I believe that surgeons ought to have the same image quality in order to get the best results.”
In the preoperative and postoperative setting, ultrasound and other sophisticated imaging modalities such as CT or MRI are widely available. Nollert said: “Intraoperatively, however, imaging was limited to the use of C-arms (2D fluoroscopy) in the past. Therefore, one available solution was to enable the superimposition of the preoperative images with the intraoperative images. Using preoperative CT or MRI, this then creates the 3D road map that interventionalists could use for very sophisticated interventions.
“Another possibility was just to use intraoperative cone beam CT, and we are getting close to conventional CT quality with this. The elegance of this solution is that the images are automatically registered to the patient and there is, on the other hand, the actual anatomy of the patient on the table that is probably not the anatomy that you have on the [preoperative] CT (because he is in a different position and may also be in a different state of hydration). We therefore believe that the most precise imaging is done intraoperatively, particularly when you have inserted wires or other devices that have changed the anatomy. This might bring up the issue of distortion/deformation of the vessels which can be quite substantial and the overlay could somehow suggest that the operator is in the right lumen, even if they are not.”
Nollert told CX Daily News that in the past, the issue with cone beam CT was one of image quality, particularly with reference to contrast resolution and that Siemens has been working on improving this aspect. “We now have new algorithms to reduce metal artifacts and also better detectors in the latest family of systems, the Artis Q. Artis Zeego also has the latest technology and the latest detectors and we have increased the contrast resolution for cone beam CT substantially. It is important to bear in mind that all cone beam CT is not equal. We have new protocols that result in the quality being much superior to regular/conventional CT. However, image quality for cone beam CT depends on reconstruction algorithms, and how you deal with artifacts and distortion, and those algorithms are widely different in the market.”
He highlighted that the Artis Zeego was a flexible, robotic system that can adjust to the table and to being used by the whole team in the operating room. The system also allows for maintenance of the sterility and environment of the operating room as it keeps the ceiling free for uninterrupted laminar flow, or use of operating room lamps, for example. “With regard to 3D capability, the Artis Zeego has some absolutely unique features and can image large volumes, fast and achieve a superior image quality,” he said.
Clinicians in the session made the point that the robotic arm with the automated motion had the drawback that the user could not always predict how the system was going to move and often found that it was quite hard to anticipate the movement.
Kirsten Zuurmond, clinical scientist, Philips Healthcare and Koen Noordermeer, Business Development Manager, Philips Healthcare, told CX Daily News: “Philips is very concerned about the quality of intraoperative imaging. In order to reduce radiation dose and contrast values, Philips offers the possibility of fusion imaging where clinicians use the preoperative CT as a navigational map during the procedure. We also offer the flexibility of working with cone beam CT, such as in emergency situations, so that it is possible to make an accurate 3D image at the intraoperative stage.
They highlighted the flexibility of positioning the c-arm Philips system within the hybrid operating room, the advantages offered by the new system Alluraclarity, in achieving significant dose reduction, without compromising on the image quality available to physicians.
3D superimposition on fluoroscopy is not yet ideal
During the Monday morning session the issue of distortion resulting from overlaying preoperative 3D images intraoperatively was discussed. A panel comprising of chairman Peter Taylor Tara Mastracci, Cleveland, USA, Krassi Ivancev and Ian Loftus, both London, UK, noted that with fusion imaging, there was no compensation for vascular distortion and that there was imprecise fusion usage for endograft positioning and cannulation of target arteries.
“One of the issues that many clinicians have with fusion imaging is its lack of responsiveness to deformation, but most believe that a fix is on the horizon,” Mastracci said. Sixty two per cent of the audience in the session also voted against the motion that fusion of preoperative datasets is ideal.
Zuurmond highlighted that clinical opinion was clearly divided on the topic with some clinicians experiencing a high degree of accuracy. She then also explained that the company was focusing on the ostia as a target in fenestrated EVAR procedures and noted that they hoped to provide the extra help that could be useful in the area.
Noordermeer then added: “We see the benefit of the current technology but are of course working on improvements for the future to make it even more accurate and applicable. We are interested in is bringing solutions that are easier to use in the hybrid operating room and are working on automating workflow steps, making access easier and providing stepwise guidance for physicians.”
Ease of use
Gregory McIff, global director, Cardiovascular Strategic Marketing and Delphine Germain, global product manager for Hybrid OR, GE Healthcare, told CX Daily News that flexibility in the operating room, ease of use for physicians and advanced image quality were optimised to the best degree that technology allowed today in the GE systems.
In response a questions about image quality not being as good as it could be at the time of intervention, McIff noted that image quality was subjective to the user. “We know from technology that a flat panel detector gives a clearer image than an image intensifier system, so you are going to see some changes in that. But when you are dealing with aortic interventions the ability of a system to provide good imaging is perhaps, adequate. You can always have better imaging—I would love to drive a Rolls Royce, but I have to make do with my Chevrolet!”
Germain said, “We definitely emphasise that the system is easy to use. You can have the best thing in life, but it has got to be accessible, not complicated to use, too. This is an area we will keep investing in: improving the ease of use. We already have systems that allow surgeons to learn fusion imaging from tableside. With regard to superimposing preoperative imaging data within the Hybrid OR, the technique is getting complicated and it is important for us to make sure that people can use it on a routine basis.”
Both highlighted that GE focused on combining the best of the hybrid operating room with the best of the advanced imaging techniques. They drew attention to the fact that the Discovery IGS 730 was not mounted either on the wall or on the ceiling but that it could move freely in the room.
“The system confers the benefit of mobile systems that can move in the room freely so that clinicians can have access to patients, and at the same time have the ability to be brought back into imaging position and have all the advantages of a fixed system in terms of imaging such as 3D imaging and 3D fusion,” they said.
CX ilegx Collaboration embraces Electronic Endovascular Education
cxsymposium2016-10-12T13:01:23+01:00This year, the ilegx multidisciplinary team, continuing in its aim to reduce the number of leg amputations, broadcast—for the first time—edited live below-the-knee endovascular procedures to the Far East, in association with Abbott Vascular. Also, as part of the ilegx programme, the King’s College Hospital Open Access System showed the latest treatment options for diabetic foot. Innovative methods of foot revascularisation were also discussed and a guidewire tutorial was taught.
The morning session titled “Electronic Endovascular Education—Edited live cases broadcast online to the Far East”, chaired by Max Amor (Essey-les-Nancy, France) and Roger Greenhalgh (London, UK) showed delegates three complex below-the-knee procedures from Germany, Italy and France.
The first case was performed by Andrej Schmidt in December 2012 in Leipzig, Germany. The second procedure was carried out by Roberto Ferraresi in March 2013 in Milan, Italy, and Eric Ducasse undertook the third case in Bordeaux, France, in March 2013. Ducasse said: “In the past decades we have seen major advances in the treatment of below-the-knee lesions with dedicated materials and retrograde approaches to peripheral arterial disease.” For this case, Ducasse showed below-the-knee techniques using Abbott Vascular materials. He showed a retrograde approach through the peroneal artery followed by guide wire proximal recapture and successful balloon angioplasty.
After each case, Dierk Scheinert, Leipzig, Germany; Flavio Airoldi, Sesto San Giovanni, Italy; Ferraresi, and Ducasse, answered questions, via video link, from delegates watching in the Far East. Questions came from Japan and India, and also from Tunisia.
At the end of the session, Greenhalgh told delegates: “This Electronic Endovascular Education session has been a wonderful experience with great educational value. I have to thank these physicians from Germany, France and Italy for those fantastic results and for sharing with us all that can be done to save legs.”
He added: “This experience forms part of the CX ilegx Collaboration Day and ilegx stands for interdisciplinary management of legs because we are concerned that too many legs are being amputated,”
Greenhalgh told CX Daily News: “In these times of economic difficulties—when flight costs are on the rise—this online educational experience could be a cost-effective way of sharing education. We would like to invite the participants of this experience to share their opinion via twitter or facebook on whether this should be a pattern to be followed in future CX meetings”
King’s College Hospital Open Access System aims to save diabetic foot
The King’s College Hospital Open Access System, London, UK, “includes a multidisciplinary team of podiatrists, nurses, microbiologists, vascular surgeons, orthopaedic surgeons, diabetologists and interventionalists dedicated to providing an urgent, immediate treatment to patients who are at risk of developing necrosis, gangrene and losing their legs,” Michael Edmonds, King’s College Hospital, told CX Daily News. “The two drivers for this—in diabetic patients—are infection and ischaemia. Rapid diagnosis of infection and rapid treatment will prevent the progression of the necrosis. At the same time, the vascular system should be addressed and we should go forward with revascularisation, either with angioplasty or bypass, depending on the degree of the circulation problem as soon as possible,” he added. Edmonds presented an update entitled “Rapid referral and treatment within the concept of the diabetic foot attack” at the CX ilegx session.
Physicians with different specialties from the King’s College Hospital Open Access System also gave presentations at the ilegx Collaboration Day on their experience treating diabetic foot as a multidisciplinary team.
Jason Wilkins, London, UK, presented a modern interventional approach to the diabetic foot. He told delegates, “The modern interventional approach to the diabetic foot begins with teamwork and recognition of patient-centred care being at the forefront of the team approach. Ischaemia with neuropathy or infection is considered an emergency and robust patient pathways are mandatory in providing timely intervention.”
Wilkins highlighted that revascularisation was a basic requirement for successful treatment and amputation prevention. “Revascularisation may be surgical, radiological or a combined approach according to the presentation and nature of disease and distribution,” he commented.
According to Wilkins, modern techniques and equipment provide the interventionalist with excellent tools for revascularisation with angioplasty, stenting and recanalisation of multiple long occlusions. He said: “The understanding and availability of modern equipment and techniques along with an effective multidisciplinary and timely approach to urgent revascularisation result in improved outcomes for our patients.”
Hisham Rashid, vascular surgeon, London, UK, presented “Distal and ultra-distal bypass: a discussion on the foot angiosomes—fact or fiction?”
Rashid told delegates that the angiosome concept was developed in 2006 by Attinger. He commented on a study—in press—that he undertook to evaluate the impact of the angiosome concept in a group of 142 diabetic and non-diabetic patients who underwent distal and ultra-distal bypass surgery for critical limb ischaemia with significant foot tissue loss. Rashid reported: “In this cohort of patients the healing and time to healing was not affected by the angiosome revascularised, but was significantly affected by the quality of the arterial pedal arch. In patients with no pedal arch, the healing was significantly slower and inadequate compared to the complete and incomplete pedal arch subgroups. However the amputation-free survival rates were similar in all groups.”
International input to limb salvage
Also, as part of the Kings College Hospital Access System programme, speakers from Europe and USA gave their views and experiences on limb salvage.
Carlo Setacci, Siena, Italy, talked on the latest guidelines on diabetic foot treatment: The Italian consensus document. Carlo Caravaggi, Milan, Italy, presented a new integrated surgical approach—based on timing—to reconstruct the diabetic foot. Christopher Attinger, Washington, USA, told delegates about surgical care of the wound with debridement and planning of amputations and reconstruction. An interventional approach to the diabetic critical limb ischaemia patients vs. non-diabetics was presented by Roberto Ferraresi, Milan, Italy. David Armstrong, Tuscan, USA, spoke on techniques to correct foot deformity by surgical means.
Revascularisation challenges
In the afternoon, Roger Greenhalgh, London, UK, chaired the session on revascularisation challenges on the treatment of critical limb ischaemia.
Frank Vermassen, Ghent, Belgium, highlighted the importance of keeping vessel patency in the long run. He said: “Sustained patency of the wound-related artery is mandatory to optimise the chance for wound healing, to avoid repeat intervention and to preserve the limb.” Thomas Zeller, Bad Krozingen, Germany, said: “Patency is necessary but not sufficient for wound healing and ultimate limb salvage.” He added, “Drug-eluting balloons may be the solution to achieve the necessary patency levels within the extensive multivessel arterial disease typical of critical limb ischaemia.”
In the discussion, the question of using more than one balloon to achieve the necessary patency in critical limb ischaemia patients was raised. However, this approach would increase costs. Greenhalgh made the point that only patients with insurance companies willing to pay for this and patients who can afford it would receive the treatment.
A completely percutaneous closure approach is feasible in most cases
cxsymposium2015-07-11T02:23:23+01:00At the Abbott Satellite Symposium, yesterday, Ian Loftus (London, UK) reviewed the data for percutaneous closure in endovascular aortic procedures, stating that the percutaneous closure approach was “technically feasible in most cases, is safe, and leads to early mobilisation and reduced length of stay”
Loftus explained that with endovascular aortic aneurysm repair (EVAR), the traditional femoral cutdown for vessel access was still used in many cases. He commented: “Studies have demonstrated groin complication rates ranging from 5% to 15% associated with femoral cutdowns, including wound infections and lymphatic leaks. This can hinder recovery and lead to prolonged length of stay.”
He added that a percutaneous approach using arterial closure devices could potentially avoid many of these complications. Loftus reported: “Previous series and a single small randomised trial have demonstrated high technical success rates and significantly shorter operation times, quicker time to ambulation, reduced length of stay and reduced overall procedural costs associated with using the Abbott Prostar XL device [for percutaneous closure].”
According to Loftus, data from a US multicentre randomised clinical trial comparing percutaneous closure using the Proglide closure device (Abbott Vascular) with standard surgical cutdown found that major access site complications were significantly lower in the percutaneous group (6% vs. 10% for surgical cutdown) and that minor complications were halved (4% vs. 8%). He added: “There was also a significantly shorter procedural time (106 vs. 141 minutes) and time to haemostasis (10 vs. 23 minutes).”
Concluding, Loftus stated: “Complete percutaneous treatment is technically feasible in most cases, is safe and leads to early mobilisation and reduced length of stay”. He commented that the complication profile of this approach was different form the open approach, but said complications could be minimised with “prudent case selection, training and careful device deployment.”
Also at the “Close with confidence” symposium, Matt Thompson (London, UK) spoke about “tips and tricks” for percutaneous closure. These tips included being aware of blood clots, the importance of being patient, and advice on accessing the common femoral artery. He said: “Getting the correct puncture site is absolutely key to a successful procedure. Ultrasound is the best way of ensuring accuracy of puncture.” He concluded that the percutaneous approach was now the default approach for all endovascular procedures at his centre (St George’s Vascular Institute, London) and they had seen an improvement in patient outcomes after adopting the approach. Thompson added there was “definitely a learning curve” with the percutaneous closure approach.
Spotlight on Bolton Medical’s Thoracic Arch Branch device
cxsymposium2016-10-12T13:01:23+01:00In a Bolton Medical sponsored event, Piergiorgio Cao, Rome, Italy, and Toru Kuratani, Osaka, Japan, both presented their experience with the company’s Thoracic Arch Branched Device. Kuratani also presented his experience at the CX Complex Edited Live Cases and Case Reports.
Cao spoke to delegates about complex cases in which deployment of the Thoracic Arch Branch device was successful using single- and double-branches. Cao showed 3D CT images that indicated the successful use of the device in single branches in five cases (including ascending and descending aorta dilatation, arch aneurysm, pseudoaneurym, and endoleak amongst other comorbidities such as diabetes and hypertension). He commented that final angiograms of these cases were good.
With the double-branch device, Cao spoke about one patient with a thoracic aortic aneurysm involving the left subclavian artery with no proximal neck posterior to the left circumflex coronary artery (the left circumflex coronary artery and the iliac artery were also very close together). He reported that this was successful but the patient had a type 2 endoleak, which was due to be treated in the coming weeks.
Referring his experience with the device, Kuratani reviewed 3,015 cases of aortic aneurysms. Of these, 2,383 had been treated with a stent graft. He also showed a video to delegates of his team performing a debranching thoracic endovascular aneurysm repair with the Bolton Medical device.
The arch branch technology (based on the Relay non-bare stent plus platform, intended for zone 0 deployments), according to Bolton Medical, incorporates a proximal clasping mechanism intended for repositioning and a V-patch inner sheath for expansion and optimal alignment.
The (single and double) branch graft is expected to be examined via data collected by the company, from further clinical experience and a trial of the branched devices from centres preforming thoracic abdominal aneurysm repair. In phase I of this analysis, the single branch device will be tested in five institutions in six cases. In phase II, the double branch device will be assessed in six cases across six institutions.
New three-stage hybrid graft reviewed
cxsymposium2015-07-11T02:23:23+01:00Heinz Günther Jakob (Essen, Germany) told CX delegates about a new three-zone aortic arch hybrid prosthesis, which has been designed to reduce ischaemic times by 50%. Jakob reported that the three-stage hybrid graft was created with the aim of reducing the rate of complications seen with the E-vita open hybrid prosthesis (Jotec) in some patients.
He said that it consists of a “descending aortic covered stent graft, followed by a non-covered stent graft for the arch, and a Dacron part for the ascending aorta” and that it has been designed to “reduce ischaemic times by more than 50%.”
The primary indications for the graft would be “sick octogenarians” or in situations where there is “dramatic malperfusion” (such as myocardial infarction, stroke, and prolonged visceral ischaemia). He reported that in a small pig study (of six pigs), there was a technical success rate of 100% and a visceral ischaemia time of 21 minutes.
Jakob concluded: “After passing all regulatory affairs with the hospital, four grafts are under construction for the first-in-man implantation.” He added at present, the graft was just designed to be implanted via conventional surgery (through median sternotomy), but said that “of course” they also planned to assess the transapical or transfemoral route as well.
LIVE from CX35: Restore II registry confirms safety and efficacy of Relay devices
cxsymposium2016-10-12T13:01:23+01:00Today, Martin Funovics (Vienna, Australia) presented data from the Restore (Relay endovascular registry of thoracic diseases) II registry, which he said confirmed the safety of the Relay and Relay NBS thoracic stent grafts (Bolton Medical) in thoracic endovascular aneurysm repair (TEVAR)
Funovics explained that Restore II was a prospective worldwide registry of 234 patients who have undergone TEVAR with a Relay device. He reported that the Relay device was available as a “clasped” version with conventional bare springs and as an unclasped version with covered springs (Relay NBS). Referring to the unclasped version, Funovics said: “This version for the first time has differential attachment modalities for the inner and outer curvature.” The inner two clasps, he commented, that are guided by long levers from behind rather than proximally. “During the opening process, these two levers can slowly and in a very controlled manner guide the inner curvature apeses to the aortic wall.” He added that the device has been specifically designed for the descending aorta.
The patients in the registry, Funovics reported, reflected the “typical TEVAR patient population”, but he added they did have a “substantial amount” of cardiovascular comorbidities compared with the typical TEVAR population.
Funovics commented that with the Relay and Relay NBS devices, the technical success rate was 95.3% and there was a very low rate of systemic complications. Additionally, the rate of mortality following the procedure was similar to other series.
He concluded: “The Restore II registry confirms the safety and efficacy of our differential detachment mode of the inner and outer curvature springs. The results were comparable, especially in terms of neurological complications, to other registries and a relative high rate of technical success was achieved.”
LIVE from CX35: STABLE shows favourable two-year results with Zenith dissection system in type B dissections
cxsymposium2015-07-11T02:23:24+01:00Joseph Lombardi, Philadelphia, USA, presented two-year results of STABLE (The study of thoracic aortic type b dissection using endoluminal repair) today. STABLE, he said, is a prospective, non-randomised, multicentre clinical study conducted at investigational sites in Europe, Australia, and the United States to demonstrate the safety and effectiveness of the Cook Zenith Dissection Endovascular System in the treatment of patients with type B aortic dissection.
Developed as a less invasive alternative to open surgical repair and specifically for the treatment of type B aortic dissections, this system comprises the Zenith TX2 Thoracic Aortic Aneurysm Endovascular Graft with Pro-Form and the Zenith Dissection Endovascular Stent. “The combined use of proximal stent-graft and distal bare stent components provides a means to seal the primary entry tear and support the necessary length of dissection without risk from coverage of branch vessels,” Lombardi said.
A total of 86 patients (73% male, mean age 59 years) were enrolled in the STABLE study. More than half of the patients (64%) were treated in the acute phase (within 14 days of symptom onset) and a majority (73%) had presenting symptoms of impending aortic rupture and/or branch vessel malperfusion.
Lombardi, global principal investigator of the study, presented study results through 24 months, reflective of data received as of March 2013. The overall 30-day mortality rate was 4.7% (4/86), and Kaplan-Meier estimates of patient survival were 88% at 12 months and 85% at 24 months. During follow-up through two years, five patients experienced aortic rupture and no patient required conversion to open repair. There were seven cases of stroke (six cases within 30 days) and one case of paraplegia (within 30 days). Renal failure occurred in nine patients (none required permanent dialysis) and retrograde dissection occurred in seven patients (two patients died; four underwent re-interventions). Aortic remodeling, indicated by an increase in the true lumen size and a decrease in the false lumen size, was observed in both the descending thoracic aorta and the distal abdominal aorta.
“These results continue to indicate favourable clinical and anatomic outcomes with the use of a composite TEVAR construct. Follow-up through five years is ongoing to assess long-term effectiveness of this treatment strategy,” Lombardi stated.
CX35 experts sharply divided on type II endoleak challenge
cxsymposium2016-10-12T13:01:23+01:00The management of type II endoleak provoked a whole host of opinions among experts at CX35 yesterday. Are type II endoleaks benign, or not? Are “dangerous” type II endoleaks really misdiagnosed type I or type III endoleaks? Do type II endoleaks need treatment (and how), or is leaving them akin to “leaving a baby on a railway line”? Experts taking part in a panel discussion about the management of type II endoleaks following endovascular aneurysm repair (EVAR) did not agree
According to Hence Verhagen (Rotterdam, The Netherlands), type II endoleaks by themselves are benign and there is no proof that type II endoleaks cause type I or type III endoleaks. He said: “With a type II endoleak, it may be the outflow vessel that you are looking at instead of the inflow vessel [ie, it is the result of a type I or type III endoleak].”
Verhagen claimed that there “was no need to worry” about the treatment of type II endoleaks because they were associated with low pressure. He added that even if they were treated, there was little evidence that treatment would be effective—“In 100 patients with a type II endoleak, only two to five have a growing sac. Of these, only 30% will be successfully treated and that is the rate reported with experienced centres; therefore, only one of 100 patients will benefit from treatment.
However, Jean-Pierre Becquemin (Créteil, France) disagreed and commented: “I am really convinced that sometimes if you wait for more than five years to intervene, that type II endoleaks will lead to sac enlargement and maybe a type 1 endoleak.”
Becquemin argued that certain patterns of type II endoleaks were not benign and “must be treated by all appropriate means before catastrophe occurs”. He explained that he and his colleagues, in a recent study published in Journal of Vascular Surgery, reviewed the long-term outcomes of consecutive patients who had undergone EVAR for atherosclerotic infrarenal aortic or aortoiliac aneurysms between June 1995 and May 2010 at their centre (Henri Mondor Hospital, Creteil, France).
After a mean follow-up period of 31.3 months (range 12.4–61.4 months), 201 patients (of 700 overall) had at least one type II endoleak and these patients were at higher risk of sac growth and re-intervention compared with patients without a type II endoleak. Additionally, persistent (p<0.001) and recurrent (p=0.008) type II endoleaks were both highly predictive of sac growth as were type II endoleaks that were associated with a type I or type III endoleak (p<0.001). Becquemin reported that mortality was not increased in patients with type II endoleaks but added: “Type II endoleaks did not kill these patients because these endoleaks were treated.” Concluding the results of study, Becquemin said: “Believing type II endoleaks are benign is like believing leaving a baby on a railway line is fine”—ie, there is no immediate danger, but danger may be approaching.
In the ensuing discussion, Becquemin acknowledged that type II endoleak were not necessarily the problem per se, and that it might actually be sac growth that was the main issue. “But you cannot neglect type II endoleaks. That is my strong feeling,” he said.
Matt Thompson, London, UK, claimed that there was not a “one size fits all” answer regarding whether or not type II endoleaks were benign. He said: “I am a pretty firm believer that some type II endoleaks are dangerous and that they are going to lead to aneurysm problems with the endograft, but I think the vast majority are benign.” He added that, probably, the “biggest problem” with type II endoleaks was that some endoleaks were labelled as type II endoleaks when “in reality”, they were actually a high-pressure type I or type III endoleak. “I think that is where the confusion comes from”, he commented. In his view, multi-imaging modality was “very important” to prove that sac growth was really being caused by a type II endoleak rather than a type I or type III endoleak.
Thompson also claimed that his experience with open aneurysm repair indicated that some type II endoleaks could lead to sac expansion. CX35 programme chairman Roger Greenhalgh (Imperial College, London, UK), who was chairing the session, commented that he had also seen open surgery cases that had showed type II endoleaks to be the cause of sac expansion.
He said: “Years after a perfectly good Dacron replacement by my predecessor, an apparent second rupture occurred. When I got it controlled and opened the sac, the whole thing was being driven by what today we would call a type II endoleak, with a massive lumbar, which was forcing the sac and I had to tie it.” He added that while not all type II endoleaks would lead to rupture, he was concerned about the possibility of type II endoleaks driving sac expansion.
In his presentation, Thompson also gave an update about the Nellix technology (Endologix) for EVAR. He said that, at present, there was not much long-term data for the technology but the data so far did indicate it could represent a “paradigm shift” in treatment because of its apparent ability to reduce complications and endoleaks.
Although the panel discussion did not reach a consensus, there was a clear message from the audience—nearly 80% of delegates voted against the motion that “Type II endoleaks without sac expansion of more than 5mm per year needs intervention”. Greenhalgh commented: “By implication, some of you believe that type II endoleaks do need intervention if there is sac expansion greater than 5mm.”
Apples and oranges
Robert Morgan (London, UK) said that type II endoleaks were “heterogeneous” and that comparing the different types of type II endoleak was like “comparing apples and oranges. Some type II endoleaks are ‘more benign’ than others.” He added that in type II endoleaks, absence of mural thrombus, the size of the endoleak nidus, the presence of inflow and outflow vessels, and the size of lumbar arteries or the inferior mesenteric artery were all factors in predicting future sac enlargement.
However, he claimed that studies had indicated that type II endoleaks were associated with a low rate of rupture.
Morgan also reviewed the treatment of type II endoleaks, stating that there was not enough data to determine the direct sac puncture embolisation technique was the preferred option to the transarterial technique approach. He said: “In practice, there are enthusiasts for either technique. Logistics [in my view] favour a transarterial technique.”
According to Paolo Frigatti (Udine, Italy), preventing—rather than treating—type II endoleaks might be a valid strategy. He said that previous reports had shown: “Injection of fibrin glue alone or in association with microcoils in the aneurysm sac during EVAR can facilitate sac thrombosis and reduce the incidence of type II leaks during follow-up.”
Imaging for type II endoleaks needs improvement
Frans Moll (Utrecht, The Netherlands) said that imaging needed to be improved to detect more type II endoleaks. Improved imaging would also detect more feeding vessels for better embolisation. He said that MRI with blood pool contrast agent might give the needed improvement. However, he added: “Not all patients and stent grafts are MRI compatible and nothing of the effect of this better imaging on the outcome of EVAR is proven yet.”
Taking into account the diverging views on the management of type II endoleak, Greenhalgh said: “I wish I could say to the audience that the speakers today have made it easier, but perhaps it has been true to say, it is has been made more difficult for you. But one thing always to remember is that if you do not know the answer, clock that you don’t know the answer—so that you try to harder to find an answer.”
CX35 type II endoleaks survey
Delegates were invited to be part in a CX35 survey about the management of type II endoleaks yesterday. Please visit the BIBA MedTech Insights stand (Gallery Level) if you would like to participate.
Latest technology presented at the CX Innovation Showcase
cxsymposium2016-10-12T13:01:24+01:00Nick Cheshire and Stephen Greenhalgh, London, UK, chaired the CX Innovation Showcase today. The audience heard about innovation challenges and initial results from a series of new technologies for abdominal, thoracic, lower limb, neurovascular and venous interventions. Results for the new Crux vena cava filter were also presented.
Greenhalgh spoke to the audience about innovations and challenges to innovation in collecting data. He presented insights from the European Vascular and Endovascular Monitor, a consumption-based monitor covering 200 centres from Western Europe. He said that the advantage of a monitor approach was that it was enduring and effective in rapidly-shifting markets.
From the thoracic market, data showed that thoracic endovascular aneurysm repair remains the “gold standard” and that the current issues in the thoracic field were the size of the aneurysm, and the perceived need for a screening programme. For carotid procedures, Greenhalgh said the data indicated open surgery as the gold standard and that endovascular procedures were focused in the German and Italian markets.
“Endovascular procedures continue to grow at the expense of open surgery,” Greenhalgh commented on aortic-iliac procedures and femoropopliteal, with the use of drug-eluting balloons on the increase in the latter. Hefsyv identified the key trends in the market and stated that, “The biggest challenge is doing this in a cost effective way”.
In a following presentation, Caroline Hough, London, UK, spoke about usage and attitudes as customised studies. She said that customised research provides answers to specific questions such as the effect of a new market entrant, understanding purchasing decisions, factors impacting competitiveness of a product vs. the competition. She added that the research is tailored to the client and can be based on specific demographics and on qualitative and quantative research. She also noted that qualitative data work by “drawing out themes”.
She presented a case example of how targeted quantitative analyses addressed the type II endoleak challenge at CX35. The aim was to understand the current level of knowledge of type II endoleaks and to assess prevalence of treatment and preferred treatment protocols. The methodology was to target a global audience of physicians attending CX35 face-to-face and with a hard-copy quantitative questionnaire.
The results will be collated and edited highlights will be published in Vascular News and Interventional News.
Crux vena vena cava filter
Among the devices that were featured at the CX Innovation Showcase, Andrew Holden, Auckland, New Zealand, presented the results of a venous innovation—the Crux vena cava filter. He explained that the Retrieve trial was a prospective, single-arm study which enrolled 125 patients with a primary endpoint of clinical success (technical success and freedom from pulmonary embolism, migration, and device-related adverse events at 90 days).
Holden reported the implant technical success, femoral approach and jugular approach in 123 patients (98%), 106 (84.8%), and 19 (15.2%) respectively. Retrieval results were retrieval success (53/54, 98%), femoral access (38/54, 70.4%), and jugular access (16/54, 29.5%), respectively.
He concluded: “Implant and retrieval of the Crux Filter have been performed safely in the Retrieve study with high clinical success of 98%. The efficacy results are good with no reported filter migration, tilting or embolization and 2.4% pulmonary embolism rate.”
Simultaneously to the presentation at the CX Innovation Showcase, a paper on the RETRIEVE trial was published online in the Journal of Vascular and Interventional Radiology (JVIR) and is now available with open access on www.jvir.org/webfiles/images/journals/jvir/RetrieveTrial.pdf
Innovations in the neurovascular field were also addressed. The Solitaire FR intracranial clot retrieval device (Covidien) and its data were presented by Kyriakos Lobtesis, London, UK.
Legflow drug-eluting balloon
On lower limb innovations, Jean-Paul de Vries, Nieuwegein, The Netherlands, spoke about the Legflow drug-eluting balloon (Cardionovum) for the treatment of superficial femoral artery occlusions.
De Vries said that drug-eluting balloons can be an attractive alternative to stents because they do not leave any inflammatory triggering scaffolds in the artery and can be used in challenging femorocrural arterial segments. He said, however, that drug-eluting balloons vary by coating, formulation of the drug, and the elution excipients. All coatings have an influence on the efficacy of the drug delivery into the arterial wall and on treatment outcome, he added.
De Vries noted that the Legflow drug-eluting balloon has embedded paciltaxel underneath the surface as well as inside its shellolic acid drug-release matrix, which is coated onto the balloon surface to minimise embolization risk and wipe off.
He said that preclinical studies have shown that a short inflation time (60 to 90 seconds) is sufficient to inhibit smooth muscle cell proliferation, with sustained anti-proliferative effects for up to 150 hours according to unpublished data by Renu Virmani, Gaithersburg, USA.
Recently, the RAPID trial (Randomized trial of Legflow paclitaxel-eluting balloon with stent placement vs. standard percutaneous transluminal angioplasty with stent placement for the treatment of intermediate [>5cm] and [<15cm] and long [>15cm] lesions of the superficial femoral artery) started to recruit patients. It is intended that this Dutch multicentre, patient-blinded trial will enrol 176 patients.
The primary endpoint of the RAPID trial is to assess the difference in absence of binary restenosis rate between the coated and uncoated group after two-year follow-up.
De Vries reported that, to date, 30 patients have been randomised and followed by the data monitoring and safety board. No serious adverse events have been documented. Interim analysis is intended to be performed when the first 60 randomised patients have completed six months of follow-up. Follow-up assessments are intended to be performed at one, six, 12, and 24 months and include physical examination, ankle brachial indices, toe pressure measurements, treadmill tests, and duplex ultrasound imaging. First results from the RAPID trial are expected at the end of 2013.
Stanza bioresorbable scaffold
In a subsequent presentation, Holden also presented data from the Stance trial which is a prospective, single-arm, multicentre trial of the Stanza scaffold in patients with symptomatic atherosclerotic disease in the superficial femoral artery. The Stanza scaffold, which fully resorbs in about one year, is the first fully self-expanding bioresorbable technology being developed for treatment of superficial femoral artery lesions, according to Holden. The Stanza scaffold uses a conventional retractable sheath delivery system and is currently being tested in the trial in lengths up to 100mm.The primary safety endpoint is major adverse events at six months. Secondary performance endpoints include vessel patency at three, six, 12 and 24 months. In the first cohort of 25 patients, both technical and procedural success were achieved in 24 of 25 subjects with only one subject leaving the procedure with a residual stenosis of greater than 30%. There were no subjects that had a major in-hospital adverse event. The Stanzascaffold has been demonstrated to have excellent mechanical integrity with the ability to improve post-percutaneous transluminal angioplasty residual stenosis, Holden said.
In the presentation he commented that the Stance optical coherence tomography (OCT) substudy provided important information on scaffold deployment, structural integrity, and resorption, as well as underlying plaque morphology. The OCT substudy enrolled 16 patients; eight patients have been treated at Auckland City Hospital. Post-procedure OCT demonstrates good vessel wall apposition and tissue encapsulation at follow up of the Stanzascaffold. Additionally, lumen eccentricity is observed post procedurally and shifts to a concentric lumen which is sustained through 12-month follow-up suggested favourable remodelling of the vessel.
The Stance trial has demonstrated the feasibility of a fully self-expanding, bioresorbable scaffold, Holden said. To date, Stanzahas been successfully implanted with no evidence of fracture at follow-up. The combined OCT, angiography, magnetic resonance angiography (MRA), and duplex ultrasound at 12-month follow-up supports the long-term biocompatibility of the Stanzascaffold during the active resorption period. Enrolment in the Stance trial is ongoing and is expected to conclude in 2013.
At the end of the session, the result of the CX Innovation Showcase Dragons’ Den was revealed. Chris Underwood, Manchester, UK, from ESP Technology, who presented on “Creating self-sealing ePTFE vascular access grafts”, was named the winner.
Successful initial results for Treovance, even in challenging anatomies
cxsymposium2016-10-12T13:01:24+01:00The latest updates on devices for abdominal aortic aneurysms, and a discussion on the impact of going low profile so as not to compromise on device performance, formed the mainstay of a session yesterday. There were presentations on the latest results from promising new low profile devices including the Treovance abdominal stent graft (Bolton Medical), which is being launched at CX35
Matthew Eagleton, Cleveland, USA, began the session by presenting the successful initial results seen with the Treovance device as seen even in challenging anatomies. The results were from a meta-analysis of the phase I ADVANCE and BENEFIT clinical studies.
Eagleton explained that the ADVANCE trial was a prospective, multicentre, non-randomised study carried out at five European centres with 30 patients. Similarly, the BENEFIT trial was a prospective, multicentre, non-randomised study carried out at six US centres, also with 30 patients.
Treovance is a three-piece endograft system that has a novel fixation system with redundant sealing design. It is a low profile delivery system,” he said.
“Twelve-month results of the Treovance abdominal stent graft show successful initial results, even in challenging anatomies. Thirty per cent of BENEFIT patients presented with 61-75 degrees of infrarenal angulations. One year follow-up results showed that there was 100% graft patency in the BENEFIT study and 95.2% patency in the ADVANCE study. Incidence of type I, III and V endoleak was 0% in the BENEFIT study and 4.8% (type I) in the ADVANCE study. There was a 7.1% incidence of type II endoleak in the BENEFIT study and 23.8% incidence in the ADVANCE study. There was no migration or wireform fracture in either study,” Eagleton told delegates.
With regard to demographic information for both sites, the mean age was approximately 72 years and comprised mainly of Caucasian males, with the typical demographics of those presenting with aortic aneurysmal disease. He said: “A little bit of difference in the two sites was that in the US site, there were more patients that presented with diabetes, occlusive disease and neurological complications prior to insertion of the stent graft.”
Speaking on the results, Eagleton said: “In terms of acute deployment, or how feasible delivering the system was, there was 100% successful introduction, deployment and 100% stent graft patency. In addition, there were no type I, III, or IV endoleaks, no aborted procedures and no conversions to open surgery. The procedure took a little over 100 minutes with a minimum of blood loss. The hospital stays were approximately two days in the US trial and three days in the European trial.”
He also noted that at 30 days, the mortality rate at was zero in both groups and major morbidity related to these categories was also zero.
“Procedure-related adverse events were zero in the US trial and 6.7 in the US trial. One subject experienced left buttock claudication deemed serious and procedure‐related. Another subject in the BENEFIT trial exhibited urinary retention that was deemed serious,” reported. There were no device‐related serious adverse events reported within 30 days for either study,” he said.
“Beyond 30 days, two serious device-related adverse events were reported in each trial (6.7% each). In the ADVANCE Study, there were two episodes of limb thrombosis and in the BENEFIT study, there were two episodes of thrombus formation in the limb.
Eagleton expanded on the unique redundant sealing system of the device which was first used in the RelayTEVAR system and is due to an overlap of the first two. “This system may enhance sealing in even in tortuous necks. Based on this design, the device is indicated for neck lengths of 10mm with an infrarenal angle of less than 60 degrees and in 15mm or greater with an infrarenal angle between 61 degrees and 75 degrees,” he said. He noted that the infrarenal barbs provided supplemental fixation in angulated anatomies and the suprarenal barbs provided primary proximal fixation. “Multiple fixation points provide migration resistance,” he said.
“Treovance provides a versatile solution in a low profile delivery system with an accurate deployment. The device received CE mark certification on February 28, 2013 and a phase II US trial is set to commence mid-2013,” Eagleton concluded.
Also presented at the session were the results for Zenith TX2-LP (Cook Medical) and data from the PYTHAGORAS trial, which used the Aorfix stent graft (Lombard Medical) in highly tortuous aortic and iliac anatomy.
Bolton Medical symposium
There will be a lunch symposium (12.30 to 14.00pm) today at the Red Learning Centre. The symposium entitled “Bright ideas for aortic endovascular solutions”, and it will be chaired by Roberto Chiesa, Milan, Italy and co-chaired by Colin Bicknell, London, UK. Vincent Riambau, Barcelona, Spain will present 12-month results of Treovance abdominal stent graft to start the symposium.
Developments in imaging go under the scanner at CX35
cxsymposium2016-10-12T13:01:25+01:00Identifying the various strengths and limitations of the state-of-the-art imaging systems was at the core of Sunday’s morning session.
CX chairman Roger Greenhalgh said: “It is imagined that many of the post-operative problems of EVAR and TEVAR could relate to inadequate preparation and imaging. At CX35, there are three full-sized imaging suites from GE Healthcare, Philips and Siemens because imaging in three dimensions before the deployment of devices is crucial to getting the size right, meaning the central luminal line and properly measuring neck lengths, landing zones and establishing whether there is thrombus and calcium, to avoid endoleaks. So whatever the specialty—vascular surgery, interventional radiology or interventional cardiology—knowledge of imaging is crucial. There is also a need for simulation and rehearsal.”
A panel comprising of Nung Rudarakanchana, London, UK, Lieven Maene, Aalst, Belgium, and Stéphan Haulon, Lille, France, discussed the advantages of using hybrid imaging suites in the diagnosis, planning and intra-operative imaging guidance of minimally-invasive endovascular procedures. Janet Powell, London, UK, chaired the session.
The panel told delegates that vascular surgical training needed to ensure that young surgeons learn how to use 3D workstations. Haulon made the point that with complex new techniques that take time to perform, there was significant radiation exposure to both patient and operator. “We need to use every available tool to reduce the radiation dose,” he said.
Maene, agreeing, also highlighted that trainees needed to be aware about all different techniques. “You need to know about fusion imaging, cone beam CT and intraoperative marking to get the best out of all these systems. It is important to reduce the radiaition and also not lose important information for your procedure,” he noted. Rudarakanchana added concurrent advances in simulation and hybrid suite imaging now also allowed for whole teams to be trained with a view to reducing radiation dose and improving procedural outcomes.
Advanced hybrid suite imaging is the key to surgical advance
“Advanced hybrid suite imaging is said to be on key to surgical advance, allowing us to improve the quality, effectiveness and efficiency of the treatments we offer to patients with the long-term goal of improving patient outcomes,” Rudarakanchana told delegates.
“The rise of catheter-based procedures and minimally invasive surgery has led to the evolution of hybrid suites, which combine state-of-the-art imaging with the sterility of an operating theatre. These provide fertile ground for surgical innovation and opportunities to expand treatment possibilities,” she said.
“Three-dimensional image guided navigation is now possible and further innovations in robotics, advanced endoscopic vision and precise manipulation are on the horizon.”
She explained that hybrid suites accomplish imaging purposes via fixed C-arms, either floor or ceiling- mounted, which incorporate digital flat panel detectors for high image quality, large fields of view and, in some cases, three-dimensional imaging with soft-tissue contrast resolution.
Recent developments in hybrid suite imaging, Rudarakanchana commented, have now expanded capabilities beyond the traditional two-dimensional fluoroscopy and three-dimensional rotational angiography, to enable acquisition of CT-like three-dimensional imaging for image-based guidance and intra-operative functional imaging such as flow analysis.
Referring to long-term endovascular aneurysm repair (EVAR) outcomes, Rudarakanchana highlighted that “advanced hybrid suite imaging may be key to improving optimal sizing and more precise graft deployment and immediate quality control in a sterile environment. Accurate deployment of endovascular grafts and optimal stent positioning can be expected to reduce the risk of endoleaks and other complications, leading to a sustained benefit in terms of aneurysm-related survival in patients undergoing endovascular repair.”
She also noted that a wide range of surgical specialties, including cardio-thoracic, trauma, orthopaedics, urology, neurosurgery, gynaecology, maxillo-facial and hepatobiliary surgery could benefit from hybrid suite imaging facilities.
Optimal imaging and planning for abdominal aneurysm
Lieven Maene, Aalst, Belgium, explained that 3D endovascular guidance had multiple benefits in preoperative and intra-operative EVAR management with direct impact on device deployment, as problems with parallax and distortion, sizing and navigation remained areas of concern.
The SiemensArtis Zeego Endovascular Guidance system, Maene noted, had multiple benefits: precise endovascular action; control in allowed interactive imaging during EVAR with precise endovascular action; control in 3D; parallax quantification; evaluation of distortion and aortic changes; and limited use of contrast.
“3D imaging can become a vital part in our daily life, at home and in our professional life, if we give it a chance,” he said.
Maene highlighted that computed tomography angiography (CTA) delivers accurate measurements and 3D reconstructions. Also, magnetic resonance angiography (MRA) reduces radiation and nephrotoxicity, volume rendering with centerline calculation and stretch-views eliminate angulation errors. However, he said: “The most crucial phase in EVAR, the accurate deployment of the stent graft, is often poorly controlled with 2D fluoroscopy.”
To overcome this problem, Maene commented: “The use of a hybrid operating room with 3D imaging and perioperative guidance allows the physician to evaluate the patient’s anatomy in a new dimension.” Intra-operative systems such as the 3D syngo Dyna CT (Siemens) are able to reflect the possible anatomical changes and distortion due to the large-bore devices and stiff wires.
Maene explained that the system allows visualising perpendicular planes that can be quantified along the centerline of the aorta during the operation and used as markers (guidance ring) for accurate deployment taking into account these anatomical changes of the aorta.
“Partial stent graft deployment allows alignment of the guiding planes with stent graft markers to avoid parallax errors. Ostia of aortic side branches and vessel contours can be marked for navigation and move along in the three-dimensional images even when changing the position of the C-arm,” he said.
In conclusion, Maene told delegates: “Three-dimensional endovascular guidance offers a new perspective during EVAR performed in a hybrid operating room. Marking of the landing zones and side-branch ostia improves the accuracy of graft deployment and guides the physician through the challenging aortic anatomy.”
“This system may improve long-term EVAR results by decreasing type I endoleak, avoiding inadvertent side branch occlusion and may support diagnosis and treatment of postoperative endoleaks. Preoperative sizing at a multimodality workstation with current software tools remains very helpful,” he added.
Benefits of Innova Vision Technology with first GE Discovery experience
Stéphan Haulon, Lille, France, shared his experience using the GE Discovery IGS730 at Lille University Hospital. He said that the hospital installed this hybrid suite six months ago and so far has performed over 200 procedures including electrophysiology, transcatheter aortic valve implantation (TAVI) and EVAR.
“In all of the EVAR cases we performed (including standard infra-renal cases and complex cases such as fenestrated and branched endografts), we used Innova Vision to fuse 3D pre-operative CT on top of the fluoroscopy images,” he said. “With this technique, we could benefit from a 3D vascular map without the need to perform any 3D rotational acquisition or additional contrast injection at the time of the intervention.”
He also explained that access to the fused image takes a few minutes including CTA images preparation and registration on the current patient position on the operating room table. “The 3D overlay then adapts to table and C-arm movement allowing patient centering and C-arm positioning without the need to shoot X-ray,” he added.
Haulon concluded: “With our new hybrid room, we are able to keep the mobility and sterility management of a mobile C-arm while benefiting from easy to use 3D imaging techniques that help us decrease contrast media injection and radiation exposure for us and for the patients.” He added, “The mobility of the system has a clear benefit for the entire team allowing us to perform a wide variety of vascular access including axillary and carotid access.”
Haulon told delegates that he did not routinely use cone beam CT in fusion imaging as the mean dose area product (DAP) was 1200 cGy.cm², which was 10% of the DAP to implant branched endgrafts for thoracoabdominal aneurysms, 50% of the DAP for TEVAR procedures and 70% of the DAP for EVAR.
In order to keep the radiation dose down, Haulon said the team’s favourite approach for 3D overlay was fusion with 2D fluoroscopy. “One of the benefits of Innova Vision technology is that it is a workflow for dummies, even a vascular surgeon like myself can do it,” he joked. He also said: “You need to have full control of the system at the tableside and, in our practice, it has helped decrease fluoro time, X-ray dose and contrast volume.”
Cordis launches Smart Flex stent at CX35
cxsymposium2015-07-11T02:23:24+01:00Cordis launched the Smart Flex Self-Expanding Stent System at CX35. The device belongs to a new generation of self-expandable stents for peripheral indications and, according to the company, is “fully connected and yet flexible.”
Laurent Granier, marketing director EMEA, Cardiology and Endovascular at Cordis, spoke to CX Daily News on the features of this new device: “The Smart Flex stent shares a unique design legacy with the Cordis Smart stent—a first generation device. This device does not have any structures that may cause the artery to be injured after some time. The integrity of the stent is expected to be preserved in the long run, meaning that it will be free of fracture, especially in the popliteal and femoral arteries—which are areas where you need this type of stents to be resistant.”
Michael Iwanicky, global marketing leader for Peripheral Stents at Cordis, said that according to the Palmaz principles for stent design, a device should have high radial force and good tissue-metal ratio, and be fully connected. “Cordis has achieved all this with this device; it is fully connected and yet flexible,” Iwanicky said. “The device has been implanted in 200 patients so far”.
Following the recent acquisition of Flexible Stent Solutions—a developer of flexible peripheral arterial, venous and biliary stents—Granier commented, “Cordis has been able to expand the Smart platform to address unmet needs in the treatment of peripheral arterial disease and extend our capabilities to develop therapeutic applications into below-the-knee and venous interventions.”
“If we talk about education, Charing Cross is for us the most important endovascular meeting for vascular surgeons and interventional radiologists in the international European science arena. This is the place where we might effectively have the best impact launching this new generation of the Smart device”. He also commented that the product would be available from June 2013.
In Europe, the device gained the CE mark approval for all peripheral indications (femoral, popliteal and iliac arteries). In the USA, the Smart Flex stent has been approved for biliary indications.
Plain old balloons and bare metal stents do not reign supreme in the superficial femoral artery
cxsymposium2015-07-11T02:23:24+01:00After listening to the latest data for the Zilver PTX drug-eluting peripheral stent, 81% of delegates at yesterday’s stent data session voted against the motion that “plain old balloon and bare metal stents reign supreme in the superficial femoral artery”.
At the session, Frank Criado, Baltimore, USA, reviewed whether plain old balloons and bare metal stents were still the first-line treatment devices for the superficial femoral artery. He said that currently, vascular physicians were “addicted to stents” because they are predictable, easy to use, and widely available. According to Criado, there are disadvantages with this approach and these include monetary costs, the risk of in-stent restenosis, fractures and the possible need for re-intervention. However, with current positive data from the RESILIENT trial and the one-year data from the Zilver PTX trial, Criado predicted that the use of plain old balloons and bare metal stents—in the next five years—will be replaced by drug-eluting stents, drug-eluting balloons, mechanical and laser atherectomy, and endoluminal bypass.
In a following presentation, Marc Bosiers (Dendermonde, Belgium) discussed the use of the Zilver PTX in long lesions in the superficial femoral artery (>15cm). He said that stent integrity decreases as lesion length increases, but added that the Zilver PTX “provides better results in long lesions than bare metal stents”. Concluding his presentation, Bosiers announced the launch of the Zilverpass (The Cook Zilver PTX drug-eluting stent versus bypass surgery for the treatment of femoropopliteal TASC C and D lesions) study, which has a primary endpoint of patency at 12 months.
Michael Dake (Stanford, USA) then presented the three-year data from the Zilver PTX (Cook Medical) drug-eluting stent clinical trial. The Zilver PTX, according to Dake, is designed for the superficial femoral artery and is approved in Europe, Japan, and the USA. He reported that the Zilver PTX trial had a primary and secondary randomisation process. According to the results, event-free survival was 83.5% in the 185 patients who received the Zilver PTX stent compared with 72.7% in 189 patients who were treated with percutaneous transluminal angioplasty alone (p<0.01). The rate of freedom from target lesion revascularisation was 84% in the 185 patients and 70.2% in the 189 patients. Dake added that, at three years, the Zilver PTX demonstrated a low fracture rate of 2.1%.
Additionally, the primary patency of the Zilver PTX was 68.7% in 184 lesions vs. 22.8% in 207 lesions with percutaneous transluminal angiography. In a comparison between the Zilver PTX stent and a bare metal stent, the primary patency was 79.6% in 44 lesions and 56.3% in 53 lesions respectively. “Three-year results support sustained safety and effectiveness,” He concluded.
Substantial improvement in functional status with bioresorbable scaffold at 30 days
cxsymposium2015-07-11T02:23:24+01:00Yesterday, at the Abbott Vascular Satellite Symposium, Johannes Lammer (Vienna, Austria) presented the 30-day data for a drug-eluting bioresorbable scaffold (Espirit; Abbott Vascular) for the management of de novo lesions in the superficial femoral or iliac arteries—the data showed that the scaffold was associated with a 100% procedural success rate and a substantial improvement in functional status.
Lammer reported that the scaffold was made from poly L-lactide, was naturally resorbed and fully metabolised, and was a hybrid between a balloon-expandable and a self-expanding stent. He added that ESPRIT I study is a single-arm, multicentre and its aim is evaluate the use of the bioresorbable scaffold in patients with a single de novo lesion in the superficial femoral or iliac arteries and who had symptomatic claudication. The trial objectives, Lammer reported, were to evaluate the safety and performance of the scaffold and the endpoints included the procedural, clinical and functional outcomes at one, six, 12 months and at two and three years.
At 30 days, in 35 patients (recruited across seven centres), there were no deaths, no scaffold thrombosis, and no target lesion revascularisations. Also, there was a 100% acute procedural success rate. Furthermore, there was a substantial improvement in functional status—Lammer commented that the percentage of severe claudicants (Rutherford category 3) dropped from 57% at baseline to 0% at 30 days. Concluding his presentation, Lammer said: “So, you can see the initial results are remarkable.”
Delegates at the Satellite Symposium also listened to Frank Vermassen, Ghent, Belgium, speaking about femoral anatomy, characteristics and challenges, and Richard Rapoza, San Francisco, USA, on evolution in superficial femoral artery technology.
Latin America comes to CX
cxsymposium2016-10-12T13:01:25+01:00For the first time, as part of the new session “CX Meets Latin America”, Latin American physicians shared their experiences of treating abdominal aortic and thoraco-aortic aneurysm with CX delegates.
At the session entitled “Pushing the boundaries of EVAR and TEVAR”, CX delegates received information on case reports from Argentina, Brazil and Puerto Rico. Frank Criado, Baltimore, USA, chaired the session with Frans Moll, Utretcht, The Netherlands, and Tulio Navarro, Belo Horizonte, Brazil, as moderators.
Mariano Ferreira, Buenos Aires, Argentina, presented a case of a 74-year-old man with a 7cm thoraco-abdominal aneurysm with distal dilatation of the aortic arch that was treated in a three-staged thoracic endovascular aortic repair (TEVAR) procedure. Ferreira said: “Due to elevated spinal cord ischaemic risk, a three-staged TEVAR procedure was planned with prophylactic left subclavian artery revascularisation.”
He explained that the patient underwent TEVAR under general anaesthetic with adjunctive spinal fluid drainage. “Through a direct right femoral access, three Gore Tag thoracic endoprostheses (40 x 140mm, 37 x 200mm and 34 x 200mm) were implanted in an overlapping proximal-to-distal fashion covering the entire length of the thoraco-aortic aneurysm (from the left common carotid artery origin to the celiac axis trunk).”
Ferreira commented that the patient did not present any complications after the procedure and was discharged home on the fourth postoperative day. He added: “Twelve days later, the patient underwent successful percutaneous proximal left subclavian plug embolization to guarantee complete sealing of the thoraco-abdominal aneurysm.” After one-year follow-up, Ferreira concluded, a CT scan revealed complete exclusion of the thoraco-abdominal aneurysm.
A case of a “Hybrid treatment of ascending aorta and arch without opening the chest wall” was presented by Tulio Navarro, Belo Horizonte, Brazil. He said: “The treatment of the aortic arch is one of the greatest challenges to the surgeon. The conventional open repair requires sternotomy and cardiopulmonary bypass. In order to reduce complications, the use of endovascular techniques has been shown to be feasible.”
For this case, Navarro described an alternative approach to ascending aorta, arch and descending aorta with off-the-shelf devices without opening the chest wall. He said: “A 71-year-old male underwent a right-to-left carotid-carotid bypass graft. Using this access, a 16x16x95mm Medtronic limb extension was placed at the ascending aorta, and then a 44x44x200mm TEVAR Valiant-Captivia endograft was also placed at the ascending aorta distal to the limb extension, via right femoral artery.” According to Navarro, the patient tolerated the procedure well and was discharged after three days, asymptomatic and with no stroke. He told the audience that after 17 months of follow-up, the patient was still alive and remained asymptomatic, with no sequels, no strokes and that all grafts were patent.
Navarro also presented a second case, which was of a 58-year-old female with prior type A dissection and who was operated on five years ago. He explained that she presented with hoarseness and chest discomfort. According to Navarro, the CT scan showed an 8cm aortic arch aneurysm affecting all supraortic vessels and chronic dissection going down below visceral aorta. She was treated the same way as the previous case. After four months of follow-up, Navarro commented, there was still remaining hoarseness but no chest discomfort and CT scan did not show any leaks. This technique, Navarro concluded: “allows the treatment of aortic arch diseases without opening the chest wall with off-the-shelf devices with good results.”
However, this alternative approach should only be performed only in high-risk patients and needs to be evaluated with long-term results and larger series.
Also, at the session, several challenging cases from Brazil were presented—endovascular treatment of complex type IV aneurysm by Pierre Galvagni Silveira, Florianopolis, Brazil; a not suitable neck abdominal aortic aneurysm treated by physician fenestrated endograft by Gustavo Puludetto, Brasilia, Brazil; an endovascular treatment of aortic pseudoaneurysm by Alvaro Razuk, Sao Paulo, Brazil; and a case of ascending aorta treated by a heart and vascular team (Eduardo Saadi, Porto Alegre , Brazil).
Criado closed the session saying: “This is a good beginning of CX Meets Latin America; we hope that this will become a feature of CX making it better and bigger with more room for participants and more speakers from other Latin American countries.”
Lack of funding for supervised exercise programmes is a global problem
cxsymposium2015-07-11T02:23:24+01:00Despite evidence supporting the benefit of supervised exercise in intermittent claudication, there is a worldwide scarcity of funds for programmes. Experts were united in their frustration that there is a global lack of funding despite the rhetoric of the need to focus more on prevention.
Delegates heard that Cochrane reviews, the highest standard in evidence-based healthcare, suggest that the key to patients with intermittent claudication living longer is for them to stop smoking, begin a programme of supervised exercise and undergo best medical therapy. Yet, worldwide there is a shortage of financial support for studies examining the benefit of supervised exercise.
Ninety per cent of the CX audience voted that they would recommend Nordic walking (used as a surrogate term for supervised exercise), and only 10% said they would not. Jonathan Beard, Sheffield, UK, said: “Why on earth is there no funding for a supervised exercise programme for arterial disease? I think this would be something for the European Society [for Vascular Surgery] or the international societies to push for in terms of health advocacy. We are a bit too focused on technical interventions. The best thing for patients is for them to exercise, after stopping smoking.”
CX chairman Roger Greenhalgh made the point that it was only after the conditions of smoking cessation, best medical treatment and supervised exercise are met that technical interventions were to be undertaken. He said, of trials that compared one technical intervention against another, that “such comparisons are not entirely legitimate as they have the same basic slant. Supervised exercise is known to be beneficial yet this is not included in the trial designs.”
Greenhalgh referred to the MIMIC trials, two multicentre randomised controlled trials which investigated whether there is adjuvant benefit of percutaneous transluminal angioplasty over supervised exercise and best medical therapy in the treatment of intermittent claudication. Investigators found that angioplasty confers adjuvant benefit over supervised exercise and best medical therapy in terms of walking distances and ankle brachial pressure index 24 months after angioplasty in patients with stable mild to moderate intermittent claudication.
Describing the situation “across the pond”, Barry Katzen, Miami, USA, told CX Daily News that while there were plenty of data to suggest that supervised exercised conferred benefits in intermittent claudication, there were currently no initiatives, and certainly no funding to study and implement this. “There is now a push from the Government towards funding preventative or less invasive medicine. A small minority of medical practitioners in the USA question whether intermittent claudication is indeed a disease, it is seen more as a quality-of-life issue. There is no currently no funding to study supervised exercise within studies or trials and I do not see a change in the funding scenario in the future,” said Katzen.
Greenhalgh described the situation in Britain colloquially as “giving the money to the doctors and then incentivising them not to spend it. Intermittent claudication is not a disease, but a milder symptom of peripheral arterial disease. It is fair to say that there is currently no funding available for supervised exercise globally, and in Britain,” he said.
Plinio Rossi, one of the legends of interventional radiology, also told CX Daily News that “supervised exercise is very important, but seen as too expensive to be funded by the state. It is clear that supervised exercise is far more beneficial that exercise advice (unsupervised); the latter just does not hit the spot.”
Beard, speaking on the topic “Nordic walking: more effective than standard exercise programmes for claudicants?” told delegates that unsupervised exercise (ie. advice) does not work. “We do know that supervised exercise programmes are more effective than angioplasty and stents, but there are problems with compliance and funding, and many countries will not fund it. How do we ensure funding for exercise therapy for patients with peripheral arterial disease? What level and duration of support is required and which regime gives the best long-term compliance?” he asked.
Chairman Frans Moll, Utrecht, The Netherlands, told delegates at the session, “In the Netherlands, the healthcare insurance companies are reimbursing supervised exercise even beyond the six months, and specifically for claudication. Are there any other countries doing this?” On finding out from the audience that Switzerland was the only other country with this practice, he said that the situation was “certainly underdeveloped. Smoking cessation, supervised exercise and best medical practice are the key to healthcare,” he said.
New magnetic resonance imaging method fingerprints tissues and diseases
cxsymposium2016-10-12T13:01:25+01:00A new method of magnetic resonance imaging (MRI) could routinely spot specific cancers, multiple sclerosis, heart disease and other maladies early, when they are most treatable, researchers at Case Western Reserve University and University Hospitals (UH) Case Medical Center suggested in the journal Nature.
Each body tissue and disease has a unique fingerprint that can be used to quickly diagnose problems, the researchers said.
By using new MRI technologies to scan for different physical properties simultaneously, the team differentiated white matter from gray matter from cerebrospinal fluid in the brain in about 12 seconds, with the promise of doing this much faster in the near future, according to a release.
The technology has the potential to make an MRI scan standard procedure in annual check-ups, and a full-body scan lasting just minutes would provide far more information and ease interpretation of the data, making diagnostics cheap compared to today’s scans, they stated.
“The overall goal is to specifically identify individual tissues and diseases, to hopefully see things and quantify things before they become a problem,” said Mark Griswold, a radiology professor at Case Western Reserve School of Medicine. “But to try to get there, we have had to give up everything we knew about the MRI and start over.”
Griswold has been working on this goal with Case Western Reserve’s Vikas Gulani, an assistant professor of radiology, and Nicole Seiberlich, assistant professor of biomedical engineering, for a decade. During the last three years, they developed the technology and proved the concept with graduate student Dan Ma Kecheng Liu, collaborations manager from Siemens Medical Solutions Jeffrey L Sunshine, professor of radiology and a radiologist at UH Case Medical Center, and Jeffrey L Duerk, dean of Case School of Engineering and professor of biomedical engineering.
A magnetic resonance imager uses a magnetic field and pulses of radio waves to create images of the body’s tissues and structures. Magnetic resonance fingerprinting (MRF) can obtain much more information with each measurement than a traditional MRI.
“With an MRF,” Griswold said, “we hope that with one step we can tell the severity and exactly what’s happening in that area.”
Other researchers have tried to use multiple parameters in MRI’s, but this group was able to scan fast and with higher sensitivity than in previous attempts, he continued. “This research gives us hope. We can see that it is possible the MRI can see all sorts of things.”
The group expects to reduce scanning time and continue to collect a library of fingerprints, over the next few years.
Case Western Reserve and UH Case Medical Center have a 31-year history of developing MRI technology with Siemens. The MRI manufacturer and National Institutes of Health supported the research.
Live from CX 34: Landslide victories for Lowell Kabnick
cxsymposium2015-07-11T02:23:24+01:00Lowell Kabnick, New York, USA, successfully persuaded 84% of delegates to support his argument against the motion “Tumescent anaesthesia is no longer a benefit in superficial vein ablation in the office.” He explained that there was no “level one evidence” for tumescentless procedures at present. His opponent (who received 16% of the vote) was Steve Elias (Englewood, USA).
Also at CX today, Kabnick won 76% of the vote in the debate “Foam sclerotherapy for truncal ablation is underused”. He was again arguing against the motion. He said: “Foam sclerotherapy is overused, it is not as efficacious as ablation, there is concern about stroke, and it is not approved by the FDA.” His opponent was Jonothan Earnshow, Gloucester, UK, who only received 24% of the vote in his bid to support the motion of the debate.
Live from CX 34: Early results of fenestrated endovascular repair in the UK
cxsymposium2015-07-11T02:23:24+01:00Rao Vallabhaneni, Liverpool, UK, presented data from the BSET study. He told CX delegates the study set out to investigate what the magnitude of early benefit from the procedure was (for instance, a decrease in death); measure how long this lasts and what the target vessel patency is.
The study included all fenestrated EVAR procedures done between 2007 and 2010. Data were included from centres that have done more than 10 procedures and branched EVAR cases were not included. Data were collected online, Vallabhaneni said.
“Three hundred and eighteen patients (of which two were unsuccessful) were treated. The mean age was 74 years (47–86). The mean aneurysm size was 65mm (46–113). Cook stent grafts were used in all cases,” he said.
The analysis showed that combined in-hospital and 30-day mortality was 4.1%. There was approximately a 7% absolute risk reduction, he noted. “The complexity of the case was not related to the risk of death; the target vessel patency was acceptable, as serious consequences of target vessel loss is rare and the broad application appears justified,” Vallabhaneni said.
Live from CX 34: Fenestrated and branched EVAR are worthwhile, majority of CX audience votes
cxsymposium2015-07-11T02:23:24+01:00On Tuesday, Krassi Ivancev, London, UK, persuaded 62% of the CX audience to support the motion that fenestrated and branched EVAR are worthwhile. In opposing the motion, Jean-Pierre Becquemin, Creteil, France, garnered the remainder (32%).
He told delegates it was important to clarify the terminology: fenestrated stent grafts were used in juxtarenal abdominal aortic aneurysms or pararenal abdominal aortic aneurysms; fenestrated or branched stent grafts were used in suprarenal or thoracic abdominal aortic aneurysms (type IV); and branched stent grafts are used in thoracoabdominal aortic aneurysms. Ivancev said the results of open repair in such aneurysms were associated with a high mortality rate.
Ivancev based his argument on the fact that the results for open surgical repair were unlikely to improve, and that EVAR was a proven concept. “Fenestrated and branched stent grafts results are equal or superior to the results with open repair,” he said.
He admitted that there was not much long-term data for fenestrated and branched EVAR, but emphasised that the mortality from the procedure was nowhere near that of open repair.
“Patients who are unfit for open repair have been treated successfully. Cost-effectiveness of the procedures, comes with skills, the more you learn to do the better you do it,” he said.
Becquemin noted that fenestrated and branched grafts were like “haute couture, very beautiful, but very expensive”.
He told delegates that in order to be considered worthwhile, fenestrated and branched endografts needed to beapplicable to the majority of patients and that patients had to have no other alternative techniques available to them. “Fenestrated and branched grafts should have a reduced mortality and morbidity compared to open surgery, have proven long-term efficiency and be cost-effective. However, none of these prerequisites are fulfilled,” he said.
While he conceded that the mortality with fenestrated and branched endografts was low, he noted that they were associated with a fairly high rate of paraplegia and re-interventions. “There are no data so far to show any proven long-term benefit of fenestrated and branched EVAR,” he said.
Multilayer stent not a breakthrough, say 56% of CX audience
cxsymposium2016-10-12T13:01:25+01:00On Monday, CX delegates heard early data on the multilayer stent. In the discussion it became apparent that MARS requires firm thrombosis in the multilayers and flow to occur into the branches. The indicator of success is the reduction of sac diameter and the consensus was not to use the device in ruptured aneurysms. It became clear that the many experts cautioned not to expect too much of the device and to restrict its use in those high-risk patients where there is no other option.
While flow-diversion was acknowledged as a fascinating concept, the ensuing discussion revealed that there were still questions regarding hard endpoints showing the device’s benefit, bail-out strategies after implantation, and whether this technology was ready to be implanted in patients yet… To the question, “Is the multilayer stent a breakthrough?”, 56% of voters said “no” while 44% said “yes”.
Michel Henry, Nancy, France, told CX delegates that the Multilayer flow modulating stent represented an alternative to current devices to treat thoracoabdominal aortic aneurysms and abdominal aortic aneurysms. He explained that the key principles of the multilayer stent are: vortex velocity reduction, flow lamination in the collaterals, flow acceleration, shear stress reduction at the aneurysm neck. The physiological exclusion leads to the branches remaining patent, he said.
In the Moroccan experience, eight thoracoabdominal aortic aneurysms, five abdominal aortic aneurysms and three dissections were treated. Technical success was 100%, on an average between one and four devices were used. Thirty-day outcomes showed that there were no deaths, branch patency was 100% and that there were no neurological complications.
“These are good outcomes if we compare then with current endovascular procedures,” said Henry. “During the follow-up, we observed a progressive sac thrombosis and shrinkage depending on the importance of collaterals. Henry said, “Despite the severity and complexity of the cases we treated, the preliminary clinical results are satisfactory and promising. We did not observe any neurological complications (these are usually to the level of 10-15% of the cases, with current techniques).
The multilayer stent leads to progressive sac thrombosis and shrinkage depending on the importance of collaterals.” Henry told delegates “To have good results, it is important to have a perfect technique of implantation and to avoid endoleaks (type I or III.).” He made the point that the multilayer stent was not to be used for treating ruptured aneurysms or mycotic aneurysms. “Do not oversize the stent by more than 20%,” he said. He also noted that treating any branch stenosis before covering with the device was mandatory. He highlighted the importance of one month of aspirin and clopidogrel therapy, as well as the importance of an early post-operative CT scan to ensure proper device placement.
“While greater experience and larger follow-up are needed, the multilayer stent appears as a breakthrough to treat any aneurysm,” he said,” Henry said.
Charles McCollum, Manchester, UK, explained that to answer the question of how safe it was to cover side branches, his team had looked at data from Italy and France where >300 vital side branches had been covered (brachiocephalic, carotid, subclavian visceral or renal arteries). There had been one early superior mesenteric artery occlusion and onecoeliac axis thrombosis showing the safety of the stent, he said.
“In Manchester, we have implanted 47 stents and covered and they are all patent with no major complications,” he added. The first 12 multilayer stents were implanted by McCollum’s team in iliac or popliteal aneurysm, with follow-up ranging from 4–16 months. “Eleven fully thombosed to stent (median one week) and there were two revisions. All internal iliac arteries remained patent and there was no aneurysm growth. Three aneurysm sacs shrank >4mm,” he said.
“We also did eight compassionate cases and these have not had a good outcome”, noted McCollum. Among these cases, there was one mycotic thoracoabdominal aneurysm (sepsis at five months) and one 10.8cms suprarenal abdominal aortic aneurysm (rupture at two months). “This stent is not a miracle worker.”
He also said “We have developed a protocol to do 40 perirenal or thoracoabdominal aneurysms where the aneurysm involves or is very close to the important vessels either proximally or distally. These patients are not fit for open repair and standard EVAR is not possible, but they still have a life expectancy >12 months.” McCollum said the investigators had been measuring the patent sac diameter and had seen that the peripherals thrombose very quickly to the aneurysm, but that the abdominals were taking longer. “At this stage before we do a proper clinical trial, we have to see that there is no rupture and that the aneurysm begins to shrink, before you could possibly say this technique is successful.”
Thomas Larzon, Örebro, Sweden, who presented the Örebro experience told CX delegates that the multilayer stent approach is not applicable for all types of aneurysms. “Ruptured aneurysms are contraindicated, but the technology might be applicable in a sub-group. There are no hard endpoints that support that it really works, but its use can be justified in compassionate cases,” said Larzon.
Investigators in Örebro began a study in November 2010 and treated 13 patients with aortic aneurysm. All of these were compassionate cases. Seven of these 13 were non-symptomatic and four were symptomatic. There were also two cases of rupture. Eleven of the 13 were thoracoabdominal aneurysms. Six patients had had previous aortic surgery. Follow-up CTA was every third month and investigators recorded overall/aneurysm-related death, aneurysm size, branch vessel patency, thrombus formation, re-intervention and major adverse events.
“Three patients died due to non-aneurysm related causes (two from cardiac infarction and one from unknown causes). However, two other patients clearly died from the aneurysms, one from a ruptured aneurysm and the other had a very rapidly expanding aneurysm (which was probably a mycotic aneursym) and she died two days after the intervention,” Larzon said. In terms of size, the investigators observed that there was no decrease in aneursym size, and that this was over 15-month follow-up period. In fact, two patients had a significant increase in size,” noted Larzon.
“No thrombus was observed in three cases; there was partial thrombus in four cases and total thrombus formation in one case,” he added. Larzon told delegates that in terms of vessel patency, 28/29 of the major branch vessels were patent. A lumbar occlusion was also identified. The team also observed a spinal infarction with paraplegia (at 15 months).
CREST fails to give carotid stenting a boost at CX
cxsymposium2015-07-11T02:23:24+01:00Ninety three per cent of the delegates attending the carotid sessions yesterday voted “no” to the question “CREST is telling us to swing towards carotid stenting, Should we?” at the great debate. The results of the voting gave Michael Jenkins and Michel Makaroun clear victory over Sumaira Macdonald and William Gray Sumaira Macdonald, Newcastle upon Tyne, UK, said that CREST was one of the largest contemporary randomised trials of carotid artery stenting versus carotid endarterectomy in standard risk patients.
“It forms part of the ‘big four’ representing the current evidence base for carotid intervention in ‘standard risk’ patients the remaining trials being EVA3S, SPACE, and ICSS,” she noted. “Whilst all are important in terms of current world knowledge, a number of process issues have arguably limited the generalisability of the other three. These ‘issues’ include failure to mandate the use of embolic protection devices and adequate baseline experience in the carotid artery stenting limb of the European trials, suboptimal myocardial infarction surveillance and myocardial infarction adjudication and for EVA3S and SPACE, and early termination.” Macdonald stated that CREST was exemplary by comparison: “The use of embolic protection devices was mandatory, the interventionists were properly trained, the incidence of periprocedural myocardial infarction was carefully screened and this outcome stringently adjudicated. It ran to completion. It is perhaps one of the more robust bodies of evidence, therefore and as a result, plays an important role in the provision of recommendations,” she said.
Siding with Macdonald, William Gray, New York, USA, told delegates that the CREST data clearly tells us to “swing” toward stenting as a reasonable alternative to endarterectomy. He noted that in CREST there are important findings beyond the primary endpoint. “There were slightly more minor strokes in the stenting group and slightly more myocardial infarctions in the endarterectomy group. The long-term consequences of these two disparities had distinctly different implications: by six months, the neurological status of both endarterectomy and stenting patients with minor stroke had equalised, and there was no mortality differential. At four years, one in four patients who sustained a myocardial infarction of any magnitude had died, as compared to an incidence of one in 10 deaths among the patients who did not have an event, a statistically significant difference,” Gray said.
He added that the age differential that was first published in the New England Journal of Medicine based on the intention-to-treat analysis and suggested that there was an advantage to endarterectomy over the age of 80 “was actually incorrect, as shown by the per-protocol analysis performed by the FDA in its assessment of the data”. “In point of fact the hazard ratio of the two techniques was not different (1.01) over the age of 80, and actually only showed a significant difference under the age of 60 with an advantage going to stenting in that age group,” he noted.
Gray also said that, although CREST included both symptomatic and asymptomatic patients, subsequent pre-planned analysis showed no differences for the primary outcome regardless of the symptom status. “There were non-primary outcome ‘nuisance’ events favouring stenting. There were no cranial nerve injuries in the stenting group, and over 5% in the endarterectomy group, more than 2% of which persisted at six months and almost of which involved a motor deficit. There was an approximately 8x greater incidence of access site complication for endarterectomy,” he said. “Based on these facts and analyses the unbiased observer, but more importantly the well-informed physician, cannot exclude stenting performed by an experienced operator using embolic protection in a well-selected patient as a reasonable, and in some instances, preferable as less invasive, therapy for patients requiring carotid intervention,” Gray said.
He added that “since there has been a decade-long controversy regarding the place of carotid artery stenting, with the vascular surgical specialty community largely opposed to the concept and practice, it is now fair to state that CREST causes us to ‘swing’ away from this nihilistic approach and toward parity between the two therapies. Used in such a complimentary fashion, we have the opportunity to provide the lowest risk alternatives to our patients, who are the ultimate beneficiaries of our ‘swing’.”
CREST has been misinterpreted
Representing Frank J Veith, New York, USA, who was not able to make CX 34, Michael Jenkins, London, UK, said that as a randomised controlled trial, CREST was in many ways “exemplary”. However, he added, like most randomised controlled trials, CREST had flaws and weaknesses. “It can also be argued that CREST results have been somewhat misinterpreted by its authors to reach unjustified conclusions. More importantly, CREST results have has been further misinterpreted or ‘spun’ by others to produce conclusions that are unjustified by the data from the trial,” Jenkins said. For example, he continued, CREST formed the basis for an important part of the American Heart Association Guideline on Management of Patients With Extracranial Carotid and Vertebral Artery Disease, which was also approved by 13 other organisations. “This important guideline document reached one conclusion that ‘carotid artery stenting is indicated as an alternative to carotid endarterectomy for symptomatic patients at average or low risk of complications associated with endovascular intervention…’”
Jenkins argued that CREST was originally designed to compare stenting and endarterectomy for the treatment of moderate and high grade carotid stenosis in recently symptomatic (six months) patients. However, he said, a large number of asymptomatic patients (1,180) were also included beginning in 2005. He criticised the inclusion of myocardial infarction in the primary endpoint. “There were substantially more deaths and strokes in the stenting treated patients than those treated by endarterectomy. Only when myocardial infarctions were included were the adverse events similar in the two groups,” he said. “CREST had several additional flaws. Adding asymptomatic patients to the study diluted its power and prevented significance from being reached in some of the adverse events. The composite endpoint weakened the trial. Myocardial infarctions are not the equivalent of strokes. This is borne out by the greater degree of disability after a stroke than a myocardial infarction. There is another possible flaw in CREST. The stenting treated patients received more intensive antiplatelet therapy during and after their procedure than did the carotid endarterectomy patients,” he added.
Jenkins also noted that in CREST “the stenting operators were only vetted to entre patients after they were shown to have a high level of experience and skill. Thus the stenting results in CREST may not be representative of those generally performing the procedure in the ‘real world’. This possibility is supported by the higher adverse event rates with stenting in all reported population based studies than in CREST.”
“What about the American Heart Association Guideline and its conclusion, largely based on CREST, that “carotid artery stenting is an alternative to carotid endarterectomy” in symptomatic average and low risk patients? A Webster’s dictionary definition of alternative is “choice between two things”. This implies equivalence. In view of the described considerations about CREST, its data details and the ICSS findings, it would seem that the American Heart Association Guideline’s conclusion that stenting is an alternative to endarterectomy is not yet justified except when there are clear contraindications to endarterectomy in a symptomatic carotid stenosis patient requiring invasive treatment. High or long lesions or infected, scarred or immobile necks represent examples of such contraindications.”
Agreeing with Jenkins, Michel Makaroun, Pittsburgh, USA, quipped whether Macdonald and Gray were “swinging for CREST or swinging for cash”.
He added that the CREST primary endpoint is flawed because it is a composite that combines periprocedural myocardial infarction with stroke and death. “CREST demonstrated that myocardial infarction has almost no bearing on patient quality of life, while both minor and major stroke clearly affect multiple quality of life domains. Without myocardial infarction, the CREST clearly demonstrates endarterectomy to be superior to stenting. Patients who suffer periprocedural myocardial infarction are indeed at a higher risk of post-operative death, but the CREST investigators clearly state that there is no cause-and-effect relationship, as the myocardial infarction is likely just a marker for more severe concomitant cardiac disease.”
In CREST, Makaroun continued, asymptomatic cardiac ischaemia was included as an endpoint, but asymptomatic neurologic events were not. “Data from ICSS suggests that nearly three times as many stenting patients as endarterectomy patients have new MR imaging lesions post-procedurally,” he said. “The CREST periprocedural results are very good, but the applicability of the stenting results to a wider population outside of the study is questionable. CREST appears to be telling us that symptomatic patients, older patients, and women are better served with endarterectomy. This leaves asymptomatic, younger male patients as having equivalent outcomes with stenting and endarterectomy. These young patients are probably not well served by a new technology with limited long term follow-up. CREST is telling us many things, but it is decidedly not telling us to swing towards carotid stenting.”
AJAX confirms no difference between EVAR and open repair for ruptured aneurysms
cxsymposium2015-07-11T02:23:24+01:00On Sunday evening, CX 34 delegates heard the results of the world’s first multicentre, randomised, controlled trial that compared EVAR and open repair for ruptured abdominal aortic aneurysms. The Dutch AJAX trial results demonstrated no difference between EVAR and open repair in emergent cases. In the same session, the audience also heard an outline of the SWIFT study on the effect of transport on ruptured aneurysm treatment outcomes. While AJAX results shed some light on treatment for ruptured aneurysms, many questions still remain unanswered
Ron Balm, Amsterdam, The Netherlands, presented the AJAX results, and Regula von Allmen, London, UK, presented on the SWIFT study (Swiss ruptured aneurysm favourable transport), which investigates whether the time from diagnosis to intervention relate to operative death of ruptured abdominal aortic aneurysm.
“Some excellent results have been reported in Switzerland which is a circumscribed country where the centres performing vascular surgery are clearly identified,” she said. Von Allmen told delegates that there are great variations between centres in the country. “Reports from Zurich show that 50% of the ruptured aneurysm patients are managed by endovascular means and 50% by open repair. Zurich reports excellent results for EVAR with a 30-day mortality rate of 13.5%; open repair has a mortality rate of 32.4%. But if patients look anatomically unsuitable for EVAR, then this may not be a fair comparison between open and endovascular repair.
“However, when we look at data from Bern, there are only 4% who are treated by endovascular means and the vast majority, 96%, is treated with open repair, and the overall 30-day mortality is 15.3%. “From this we can see that there are pockets of excellence in the treatment of ruptured abdominal aortic aneurysm in Switzerland. As a consequence, it is claimed by one centre that it is unethical to carry out treatment other than EVAR for ruptured aneurysms, but there are obvious counter claims for open repair based on the data from Bern. Disparities in views often point to uncertainty and there is no proof that surgical approach is the key,” she said.
Close on the heels of this presentation came the results of AJAX, which showed that indeed there was no difference between EVAR and open repair in the treatment of ruptured abdominal aortic aneurysms. The primary endpoint of AJAX was combined death and severe complications at 30 days. Our hypothesis was that EVAR would do better than open repair with endpoint rate of 0.40 and 0.65 for open repair, β=0.20, α=0.05 in a sample size of 112 patients. Secondary endpoints were length of hospital and intensive care unit stay, intubation/ventilation and use of blood products.
“The trial area covered 1.2 million inhabitants and three trial centres and seven regional hospitals contributed data. All patients with ruptured aneurysms in the trial area were identified and followed,” said Balm. He told delegates that the preferred EVAR technique was use of an aorto-uni-iliac graft with contralateral occluder and femorofemoral crossover bypass. “Between April 2004 and February 2011, 520 patients with ruptured abdominal aortic aneurysms were enrolled in the trial and 90% (466) were enrolled in a trial centre.”
Three hundred and ninety five patients were evaluated with CTA, and 240 were found to have unfavourable anatomy for EVAR. Thirty nine patients were excluded, of these 16 were unfit for open repair, 11 were excluded for logistical reasons, seven for haemodynamic instability following CT, and five patients refused surgery. Balm said, “116 patients were randomised: 57 to EVAR and 59 to open repair. The results showed that in terms of the primary endpoint EVAR had a combined and severe complications rate of 42% (24/57) at 30 days. In the open repair group, this rate was 47% (28/59), (ARR 5.4% [95% CI -13 to +23]). These results showed, said, Balm, that the hypothesis that EVAR is better than open repair, could not be confirmed.
With regard to the secondary endpoints, ICU stay with open repair was 48 hours while it was 28 hours with EVAR (p=0.14); hospital stay was nine days with EVAR and 13 days with open repair (p=0.57); 39 patients had to use a mechanical ventilator with EVAR while 52 did so with open repair (p=0.002). Balm said, “Blood loss with EVAR was 500 ml while it was 3500 ml with open repair (p<0.001). Forty five EVAR patients needed blood during the surgery while 56 patients did so after open repair (p=0.01).
“EVAR performed a little better on the secondary endpoints,” Balm said. He told CX delegates that death with EVAR was 21% (12/57) while with open repair it was 25% (15/59). “Was there a selection of haemodynamically stable patients, asked Balm, noting that 17% of the entire cohort was haemodynamically unstable (78/466). In the randomised controlled portion, 20% was haemodynamically unstable (23/116).” Additionally, he also posed the question of whether the triallists had selecting simple anatomy, by drawing attention to the death rate following open surgery in patients with unfavourable anatomy, which was 26% in the cohort (58/222). The 30-day death rate of all consecutive patients who underwent surgery was 30% (138/454) (95% CI 26–35%).
Balm cited data from Visser P et al that was published in EJVES in 2005. The population-based analysis showed a 41% in-hospital operative mortality in The Netherlands (95% CI 40–42%) Importantly, said Balm, ‘all comers’ were consecutively enrolled in the analysis that this was a major strength of this study.
In conclusion, Balm said, “With AJAX trial results showing that EVAR vs open repair was ARR 5.4% (95% CI -11 to +23), we saw that open repair performed much better than expected with low death rates in the randomised controlled trial, but also low death rates in the entire cohort.” He said, “This could be attributable to the introduction of the trial with optimised logistics and patient care such as the pre-operative CTA and centralisation.”
Live from CX 34: Tack-IT endovascular stapler wins New Technology Dragons’ Den award
cxsymposium2015-07-11T02:23:24+01:00First-time Dragons’ Den winner Peter Schneider (Honolulu, USA) beat off stiff competition with his recently CE-marked device the Tack-IT endovascular stapler, which is designed to optimise peripheral angioplasty results for the treatment of peripheral artery disease.
The other contenders were Ralf Kolvenbach, Düsseldorf, Germany, with the BYFix Anastomic device (HDH Medical), which is used to mechanically connect any standard vascular graft to the blood vessel; Claude Mialhe, Draguignan, France, with the Twister device - a new concept of endovascular embolisation and occlusion; and James Coleman (Dublin, London) with a novel percutaneous transapical closure device for structured heart disease and aortic arch procedures.
Schneider said that the acute technical success with the endostapler device “was good”, the ability to place the device “right where we wanted it” was 96% in their first-in-man series, and procedure time was similar to a standard lower extremity case. He added: “We got the acute stent-like result without a stent while addressing some of disadvantages of stent.”
External aortic wall diameter in screening is preferred, CX delegates believe
cxsymposium2020-01-08T13:57:27+00:00In the first debate of Sunday’s CX, the majority of delegates (71%) agreed with the motion that “we prefer external aortic wall diameters in screening”
Naghmana Riazuddin, Wycombe, UK, who was speaking for the motion, said that the inner wall of the aorta was difficult to see and that the external wall was “much clearer”.
To support her arguments, Riazuddin outlined the findings of the UK Small Aneurysm Trial, which showed that the “outer to outer” method of measuring aortic diameter (in abdominal aortic aneurysms) could be used without any problems. She added that there is approximately 3mm of difference between inner-to-inner measurements and outer-to-outer measurements.
She said, therefore, if a patient was found with an aortic diameter of 2.9cm, by using the inner-to-inner method, they could actually have a diameter of 3.2cm. Riazuddin explained that this could have implications for treatment. She said: “A 4.7cm aorta via an inner-to-inner method would not be referred for vascular surgery, but it would be 5cm via an outer-to-outer method.” The threshold for intervention is 5cm. Concluding, she said that the outer-to-outer method was traditionally used and most of the trial data was based on this method. She explained that in a poll of 41 centres across Europe, 32 said that they used the outer-to-outer method. She added: “Aortas scanned by others outside of the [UK National] screening programme are still using outer-to-outer, so will we have different actual measurements for the 5.5cm threshold?”
Arguing against the motion was Tim Hartshorne (Leicester, UK). He outlined the benefits of the inner-to-inner method. He said a study, of which he was the lead author, found that (as Riazuddin argued) there was the expected difference between the inner-to-inner method and the outer-to-outer method in terms of diameter, but it also showed that there was better accuracy and reliability with the inner-to-inner method.
He said: “This is important in the context of National Screening Programmes to assure consistency between technicians and surveillance visits.” Countering Riazuddin’s argument that the outer-to-outer method should be used because the 5cm threshold is based on trials that used that method, he said: “The inner-to-inner method should be used given that that there is evidence of better reproducibility but it would be possible to adjust referral thresholds if it was found that there was an increased rupture rate in men under surveillance, for instance from 5.5cm to 5.2cm (inner-to-inner method).”
He added: “Screening programmes including the NHS abdominal Aortic Aneurysm Screening Programme will gather large amounts of data on the natural history of aortic aneurysms, providing information and evidence that may lead to modification and improvement of the present schedules.”
After the debate, Roger Greenhalgh, Imperial College, London, CX programme chairman, and chair of the first aortic session, also posed the question: “Is it crazy that we ever adopted a variety of ultrasound borders for determining aortic diameter for infrarenal aortic abdominal aneurysms? The delegates overwhelmingly voted “yes” , with only 17% saying “no”.
CX delegates give a standing ovation to Nicholay Vodolos
cxsymposium2016-10-12T13:01:25+01:00As Nicholay Volodos, who developed a self-fixing synthetic endoprosthesis in the former Soviet Union in 1984, was unable to make CX 34, Krassi Ivancev, London, UK, spoke about the early history of endovascular repair on his behalf. Commenting on Volodos’ work, Ivancev said he was the “pioneer who started the endovascular stent graft revolution.”
Roger Greenhalgh, London, UK and CX programme chairman, also praised the work of Volodos and asked people to stand if they thought it was worthwhile sending a message, via Ukranian delegates, to Volodos about how much everyone appreciated Volodos’ achievements – the entire audience stood up to celebrate his work. Volodos used the self-fixing synthetic endoprosthesis to perform his first transfemoral remote endosprothesis implantation in an iliac artery. This was in May 1985.
Two years later, he performed an endovascular repair for an aneurysm in the descending section of the thoracic aorta. “The world’s first EVAR was performed in 1987 by professor Nicholay Volodos in Kharkov, Soviet Union, and introduced in an article written in 1988,” Ivancev said at CX.
The first EVAR procedure reported in the literature took place on 7 September 1990, when Juan Parodi, Julio Palmaz and HD Barone at the Instituto Cardiovascular de Buenos Aires, Argentina, treated an abdominal aortic aneurysm patient. The aneurysm was excluded endoluminally with a Dacron graft that was anchored at the proximal infrarenal neck with a stainless steel balloon-expandable stent. Ivancev said, “Volodos realised the mechanical properties required of a stent graft. He designed, manufactured and implemented the first stent graft and his principles are still valid and exploited by us today.”
Volodos’ early experience was recorded in a paper titled “Experience with endovascular stent grafts for arterial disease from 1985 to present”, which was presented at the 21st Annual Symposium on Current Critical Problems – New Horizons and Techniques in Vascular Surgery in 1994. He wrote: “Trying to realise more effectively Charles Dotter’s attractive idea of implanting a prosthesis in not easily approached vessels through a superficially lying vessel, we developed a self-fixing synthetic endoprosthesis in 1984. This endoprosthesis received the national patent of the former USSR on 22 May 1984. At that initial stage we called the method a remote endoprosthesis.” “The design feature of the endoprosthesis was a fixing element in the form of a radial zigzag shaped cylindrical spring. The spring was made from stainless steel wire 0.4–0.5mm in the diameter.
The height of the fixing element was about 18mm. As a rule, the number of rings was six or seven,” Volodos reported. The first animal studies with the endoprosthesis were conducted in dogs, with the transfemoral prosthesis implanted in the thoracic aorta. “The results of the study showed good function of the endoprosthesis six months after the operation,” Volodos said.
Volodos and colleagues used these results as the basis to perform endoprosthesis implantation in patients. “The first operation of transfemoral remote endoprosthesis implantation in the iliac artery in the clinic was performed on 4 May 1985.
It was done in combination with a simultaneous femorotibial bypass,” he wrote. In the first report, Volodos said that 19 patients had been treated for stenosis and occlusions of the iliac arteries. “Positive results were achieved in 17 patients immediately after operation and in 15 patients in the late period (from one to eight years).” “Transfemoral remote endoprosthesis implantation with the self-fixing synthetic endoprosthesis in case of the traumatic aneurysm of the descending section of the thoracic aorta was performed on 24 March 1987. It was performed in four patients, with positive results in all of them. At 7.5 years of follow-up, it has shown good function of the endoprosthesis.” After that, Volodos performed endoprosthesis implantation of the abdominal aorta in five patients – two of them received a bifurcated synthetic endoprosthesis. “The accumulated experience with the self-fixing synthetic endoprosthesis permits us to consider that remote endoprosthetics should have a place in the treatment of patients with lesions of the aorta and main arteries,” Volodos concluded in the paper.
Gene linked to abdominal aortic aneurysm is found
cxsymposium2020-01-08T13:56:39+00:00Matt Bown, Leicester, UK, led an international team of investigators which identified a single gene that is linked to the development of abdominal aortic aneurysm. During the discussion session, after the results were presented, the point was made that the interaction between the gene LRP1 and environmental factors was key.
Bown told CX delegates on Sunday that the investigators carried out a genome-wide association discovery study of 1,866 patients with abdominal aortic aneurysm and 5,435 controls and replication of promising signals (lead single-nucleotide polymorphism [SNP] with a p value <1×10−5) in 2,871 additional cases and 32,687 controls and performed further follow-up in 1,491 abdominal aortic aneurysms and 11,060 controls. “In the discovery study, nine loci demonstrated association with abdominal aortic aneurysms (p<1×10−5). In the replication sample, the lead SNP at one of these loci, rs1466535, located within intron 1 of low-density-lipoprotein receptor-related protein 1 (LRP1) demonstrated significant association (p=0.0042),” Bown said. “We confirmed the association of rs1466535 and abdominal aortic aneurysm in our follow-up study (p=0.035).
In a combined analysis (6,228 abdominal aortic aneurysms and 49,182 controls), rs1466535 had a consistent effect size and direction in all sample sets. No associations were seen for either rs1466535 or the 12q13.3 locus in independent association studies of coronary artery disease, blood pressure, diabetes, or hyperlipidaemia, suggesting that this locus is specific to abdominal aortic aneurysms. “Our findings suggest a mechanism contributing to abdominal aortic aneurysm formation via the LRP1 pathway, and exploration of this mechanism could provide future therapeutic approaches to preventing the development and/or progression of abdominal aortic aneurysms,” Bown et al reported.
“This study identifies a biological process that could be altered using drugs and therefore treat aneurysms, either to prevent them completely or to prevent them growing,” Bown said. “The key challenges are to identify how the protein produced by this gene causes or protects against aneurysms and then work out ways to reduce or increase the activity of the protein or pathway that this protein is involved in. The next step is to find out how the protein produced by this gene is involved in the development of aneurysms,” he noted.
He concluded that the gene was aortic aneurysm specific and that it was biologically plausible. In the discussion after his study was presented, Martin Bjorck, Uppsala, Sweden, asked Bown about the possible interaction between the LRP1 gene and environmental factors. Bown said their data was limited and not powered to detect such an interaction. Janet Powell, London, UK, commented: “The reason why we white people get aneurysms must partly be because of our environment and partly because of our genes.”
The research, funded by The Wellcome Trust, was published in The American Journal of Human Genetics in November 2011.
Is it time to reconsider abdominal aortic aneurysm screening programmes?
cxsymposium2020-01-08T13:55:58+00:00CX 34 delegates on Sunday heard a variety of reasons why it might be time to question the very foundations on which national aneurysm screening programmes are based. With a population that lives longer, smokes less and has lower cholesterol levels than in the past, it seems that people are developing abdominal aortic aneurysms later in life. Do we need to raise the age threshold for screening? With all trial evidence so far having studied abdominal aortic aneurysm in men between the ages of 65 and 74, is a policy selecting 65 as the age for screening still valid? Have cardiovascular risk prevention programmes pre-empted national aneurysm screening programmes? What is an optimum rescanning interval?
Roger Greenhalgh, London, UK, addressed Alan Scott, Plymouth, UK, as they were both instrumental in providing the evidence on the 5.5cm threshold and basis of screening and said, “We made certain decisions at the time about thresholds and about screening based on information we had at the time. We are hearing a story emerging and may have to be very flexible, and re-look at thresholds and screening intervals and find that they may not be fixed intervals. They might be sliding scale intervals,” he said.
Scott replied that at the time they had been careful to set up the rescreening intervals so that they were safe, erring perhaps on the side of caution.
National screening programmes in the UK, Sweden and Australia are observing a recent reduction in growth and rupture of abdominal aortic aneurysms in 65-year-olds. Jonothan Earnshaw, Gloucester, UK, who opened the session on Sunday at CX34, took to the podium to speak about the UK’s national abdominal aortic screening programme.
In a CX exclusive, he shared draft data showing that between 2009–2012 the programme (with 40% coverage) had screened 157,730 men. The uptake was 80.17% and 2,494 (1.57%) abdominal aortic aneurysms larger than 3cm were detected. Four hundred and four men were referred for surgery.
“Perhaps the most interesting point is we were expecting, based on data from the MASS trial, to find approximately 4% of 65-year-olds with aneurysms. In fact, we have only found 1.57%. That is still a significant number of men – we have now referred over 400 men for vascular opinion.
I cannot give you outcome data, but of the first 130 screen-detected, 129 patients had survived the intervention.” Earnshaw told delegates that the main issue in screening in 2012 was the fact that “We are not finding as many aneurysms as we thought we would. Twenty-year data from the Gloucester programme showed that the mean aortic diameter has reduced from 21mm in 1991 to 17mm in 2009. There is something happening to the 65-year-old male aorta in Gloucester. This reduction in the number of aneurysms is mirrored elsewhere, in Scandanavia and Australasia, and does seem to be real. Aneurysms do seem to be going away, which is ironic, when we are starting a screening programme.”
On the issue of cost-effectiveness, Earnshaw told delegates that ongoing research suggested that that it was likely that aneurysm screening at the current prevalence would remain cost-effective.
Anders Wanhainen, Uppsala, Sweden, who then took the stage shared similar observations about the reduction of aneursysms being observed. He told delegates that Sweden had adopted a commonly suggested screening design with a single ultrasound examination of men at the age of 65. “The national abdominal aortic screening programme has a nearly nationwide coverage today,” he said. Wanhainen said that the prevalence of abdominal aortic aneurysms in the target population was lower than expected indicating a change in the epidemiology of the disease, mainly attributed to a noticeable reduction in smoking frequency. “We therefore need to continue to monitor and assess the screening programmes already established and simultaneously evaluate alternative screening strategies,” he said.
Describing the situation in Australia, Paul Norman, Fremantle, said that experts were adopting a wait and watch approach rather than implementing a national screening programme because the magnitude of benefit from screening is small. “Mortality is falling without screening. I would question whether there is enough of a public health problem to warrant screening.”
Is the rupture rate falling?
Janet Powell, London,UK, told delegates that the rate and volume of aneurysm rupture is declining. She made the point that smoking prevalence had decreased, and use of lipid-lowering and antihypertensive drugs had increased in those who were 65+ years. “The small aneurysm rupture rate is decreasing and deaths from (and hospital admissions for) large ruptured aneurysm are decreasing. Since selection for repair and its mortality are unchanged, have cardiovascular risk prevention programmes pre-empted national aneurysm screening programmes?” she asked.
Martin Bjorck, Uppsala, Sweden, told delegates that an aneurysm screening programme should include a smoking cessation programme, promotion of exercise and healthy diet and statin treatment prior to surgery. There should also be hypertension treatment, according to standard of care, he said. “As we all know, screening elderly men for abdominal aortic aneurysm, and repair of those who have or later develop large aneurysms, reduce aneurysm mortality by between 50 and 70%. “Can we prevent growth of small aneurysms? Can we prevent cardiovascular events? Can we prolong life with secondary prevention? Unfortunately, we lack specific data on patients with screening detected aneurysms,” he said. “Data on the possible effects of statins on aneurysm growth rates are contradictory, and there is no evidence to support that growth is decreased. What is shown, however, in randomised trials, is that short-term statin treatment reduces perioperative cardiac events and mortality, by approximately 50%. Long-term statin treatment prevents events among patients with cardiovascular disease, but there are no specific data on abdominal aortic aneurysm patients.”
Simon Thompson, Cambridge, UK, speaking on behalf of the RESCAN collaboration, told delegates that surveillance intervals for small abdominal aortic aneurysms differed between surveillance programmes. “Some are based on screening trials, but there is no good evidence base/direct comparative data.” The RESCAN Collaboration analysed small aneurysms measuring 3–5.4cm which came from individual patient data over time collated from surveillance programmes/other longitudinal studies.
Data were collated from 18 studies in the UK, Europe, North America, and Australia, involving 15,471 patients followed for up to eight years. There were 228 ruptures, aneurysms were measured mainly by ultrasound (external diameters) and there was a 5.5cm threshold for surgery. Results of the analysis showed that the average growth rate is 2.2mm/year and the average rupture rate is 0.2% per year. “For each 0.5cm increase in aneurysm diameter, the growth rate goes up by 0.5mm/year and the rupture rate doubles. There is large heterogeneity in these rates between studies, which is unexplained. Women have the same growth rates but four times the rupture rates of men, at each abdominal aortic aneurysm diameter.” Thomson told delegates that the clinically acceptable surveillance intervals for men are several years for 3–4cm aneursyms and six months for 5cm aneurysms. “Intervals/threshold for surgery in women should reflect those for men with 0.5–1cm larger aneurysms, and the cost-effectiveness of different surveillance policies needs to be formally assessed,” he said.
Live from CX 34: We prefer external aortic wall diameters in screening, 71% of the delegates say
cxsymposium2015-07-11T02:23:24+01:00First time presentation of one-year results from PACIFIER reinforces role of drug-eluting balloons
cxsymposium2015-07-11T02:23:24+01:00Preliminary results from eight patients within the 12-month follow-up window of the PACIFIER trial showed significantly lower target lesion revacularisation was needed for patients in the drug-eluting balloon group vs the percutaneous transluminal angioplasty group. Similarly six-month follow-up from FREEWAY demonstrated a lower target lesion revascularisation rate after Freeway drug-eluting balloon and stent than after plain old balloon angioplasty and stent.
Michael Werk, Berlin, Germany, presented the preliminary 12-month results of the PACIFIER study, which is a randomised, multicentre trial that evaluated the prevention of restenosis with paclitaxel-coated angioplasty balloons in stenosis or occlusion of femoropopliteal arteries. Werk said that the In.Pact Pacific device (Medtronic) significantly reduced neointimal hyperplasia and restenosis rates compared with standard percutaneous transluminal angioplasty in claudicants.
He added that the six-month outcomes had shown that the drug-eluting balloon was associated with a 0.01mm late lumen loss compared with 0.65mm for standard percutaneous transluminal angioplasty (p=0.0014) – of the randomised controlled trials so far for drug eluting balloons, this is the best result for late lumen loss.
Concluding, Werk said that the final 12-month clinical results were awaited and, so far, no coating-related adverse events had been noted. He said: “PACIFIER confirms and reinforces the role of drug-eluting balloons as already seen from previous similar trials.”
FREEWAY
Presenting the six-month results of the FREEWAY study, Stephan Duda, Berlin, Germany, said the study is investigating the inhibition of restenosis by stenting and angioplasty with a paclitaxel-eluting balloon (Freeway, Eurocor) postdilatation compared with stenting and angioplasty with plain balloon postdilatation in the treatment of superficial femoral artery or PI-segment lesions.
The multicentre, open, prospective randomised trial will enrol 200 patients with stenotic or occluded de novo lesions at 15 German and Austrian centres.
The primary endpoint is the rate of clinically driven target lesion revascularisation at six months. Forty six patients have reached six months of follow-up. Duda showed cases of lesions treated with the device. The preliminary results, he said, demonstrated a low target lesion revascularisation rate of 8.3% after Freeway drug-eluting balloon and stent vs. 14% after plain old balloon angioplasty and stent at six months of follow-up.
Medtronic launches largest ever superficial femoral artery drug-eluting balloon study
cxsymposium2015-07-11T02:23:24+01:00In May 2012, Medtronic will initiate the IN.PACT Global SFA Trial, a single arm, real-world study of the IN.PACT Admiral drug-eluting balloon (DEB) for revascularisation of femoral-popliteal arteries in patients with claudication and rest pain. The study will be part of the company’s drug-eluting balloon programme which already includes eight registries and 10 randomised controlled trials.
The study, which will enrol 1,500 patients across approximately 80 centres, represents the largest study ever conducted in superficial femoral artery revascularisation. The primary endpoint is clinically-driven target lesion revascularisation at 12 months and primary patency at 12 months for the “imaging cohort” with either in-stent restenosis or long lesions >10cm.
This controlled study will have independent monitoring, corelab evaluation and Clinical Event Committee adjudication to contribute to the robustness and reliability of results. Patients will be followed up to five years.
“A single-arm study of this size offers unrivalled value in the assessment of real world population and patient subgroups characterised by either isolated or combined complexities such as long lesions, in-stent restenosis, heavy calcifications, total occlusions etc,” said Gunnar Tepe, chairman of the Study Steering Committee, at CX 2012.
Medtronic’s clinical programme with IN.PACT include:
PACIFIER
This randomised superficial femoral artery trial confirmed the effect of IN.PACT DEB to significantly reduce late lumen loss and restenosis rates at six months vs. angioplasty. With a late lumen loss rate close to zero, PACIFIER reinforced the findings from previously reported drug-eluting balloon trials on the efficacy of drug-eluting balloons in the femoropopliteal bed for symptomatic patients affected by claudication and rest pain.
DEB SFA Italian Registry
In a recent publication (J Am Coll Cardiol Intv 2012;5:331–8), Micari et al described the clinical and functional results at one year of a 105-patient superficial femoral artery series treated with drug-eluting balloons. The study investigators showed a high 83.7% patency rate obtained with IN.PACT Admiral with a remarkably low stent rate of 12.3%, associated with a significant improvement of clinical and functional endpoints including quality of life and walking capacity.
Debulking and drug-eluting balloons Initial data from Thomas Zeller (Bad Krozingen, Germany) and Angelo Cioppa (Mercogliano, Italy) also signal IN.PACT DEB to be an effective adjunctive therapy to atherectomy for the treatment of superficial femoral artery lesions, especially when complicated by severe calcifications.
At LINC 2012, Cioppa reported a 10% restenosis rate at one year from his 30 patient series whereas Zeller presented his experience on 29 drug-eluting balloon patients after atherectomy. At one year, primary patency was at 88% with combined debulking and drug-eluting balloon treatment.
DEB in in-stent restenosis
While four trials are currently ongoing evaluating IN.PACT in superficial femoral artery in-stent restenosis, initial insights for this indication were given by Stabile at LINC 2012, where he presented his experience of 39 patients with superficial femoral artery in-stent restenosis. At one-year follow-up, he reported a low target lesion revascularisation rate of 7.9%.
DEB below the knee
Further down to infrapopliteal vessels, the results from the published Leipzig DEB BtK Registry (Schmidt et al, J Am Coll Cardiol 2011;58:1105-9) show a low 27.4% restenosis rate at three-month angiography in complex and long (average 176±88mm) below-the-knee lesions and occlusions.
Results appear particularly convincing when benchmarked to an historical similar patient group treated with angioplasty. Francesco Liistro (Arezzo, Italy) presented preliminary results of the DEBATE BTK randomised trial of IN.PACT Amphirion vs. standard angioplasty for the treatment of below-the-knee lesions in patients affected by critical limb ischaemia and diabetes.
With a lesion length of 121 and 123mm respectively, restenosis (29% vs. 72%; p<0.001) and occlusion rates (14 vs. 50%; p<0.001) both showed highly significant differences in favour of DEB at one-year follow-up.
DEB in arteriovenous shunts
Katsanos (Patras, Greece) presented the results of a randomised trial of IN.PACT Admiral vs. standard angioplasty for the treatment of dysfunctional arteriovenous fistulae reporting a significant improvement in patency rates in favour of drug-eluting balloons at six months. While further clinical research is warranted and underway the unceasing, broadly emerging evidence around IN.PACT DEB continues to enforce trust and boost adoption to address the daily challenges of peripheral arterial disease. Optimal percutaneous transluminal angioplasty concept and practice is being re-evaluated and the value of “leave nothing behind” reappraised for the long term benefit of patients.
First experience with a bioabsorbable stent for the superficial femoral artery presented at CX
cxsymposium2016-10-12T13:01:25+01:00The first-in-man experience results with a new bioresorbable scaffold for superficial femoral artery lesions were presented by Andrew Holden, Auckland, New Zealand, yesterday. Holden described the Stanza Bioresorbable Scaffold System from 480 Biomedical and initial case experience from the first patients treated in the STANCE trial.
The Stanza scaffold is the first self-expanding bioresorbable technology being developed for treatment of atherosclerotic disease in the superficial femoral artery, Holden said. He added that “the scaffold design is a composite structure of strong PLGA fibers in combination with an elastomer. This design enables the scaffold’s key attributes of flexibility and radial stiffness, similar to self-expanding metallic stents. Preclinical animal studies demonstrate biocompatible resorption of the scaffold over six to 12 months. The scaffold, which uses a conventional retractable sheath delivery system, is being tested in the STANCE trial in target lesions up to 100mm in length and diameters between 4.6–6mm.”
The STANCE trial is a prospective, single-arm, multicentre trial of the Stanza scaffold in patients with symptomatic atherosclerotic disease of the superficial femoral artery. The primary endpoint of the trial is major adverse events at six months, with evaluation of vessel patency and other functional and quality of life metrics assessed three, six, 12 and 24 months post-procedure. 480 Biomedical expects to enrol up to 60 patients in the STANCE study at sites in New Zealand, Australia and Europe by the end of 2012.
Holden told delegates that, in the Auckland City Hospital experience with nine patients treated to date, 100% procedural success was achieved with the Stanza scaffold. He noted that the scaffold was accurately deployed in the targeted superficial femoral artery lesion and blood flow was restored to the leg as evidenced by angiography and Doppler ultrasound.
“At Auckland City Hospital, treated vessels were also imaged using optical coherence tomography (OCT), a modality that has been used in coronary interventions but never before used in clinical trials for superficial femoral artery treatment. Post-procedure OCT images showed good vessel wall apposition with the Stanza scaffold. Additional OCT and MR angiographic analysis of the treated area will be conducted at six and 12-months to enable visualisation of the scaffold as it dissolves over time,” he said.
Is the task for TASC III consensus too great?
cxsymposium2016-10-12T13:01:25+01:00Preparations for TASC III, which seeks to achieve transatlantic and interdisciplinary consensus, are underway. Saturday’s plenary session at CX 34 saw experts from a variety of disciplines thrash out the challenges that beset TASC IIb, and outline what could be achieved with its successor. Some speakers taking to the podium challenged the notion of whether these guidelines are based on scientific evidence or expert opinion, while others questioned the validity of anatomical treatment recommendations as well as discussed how to balance the importance of consensus in the absence of high-grade scientific evidence. Johannes Lammer, Vienna, Austria, and Roger Greenhalgh, London, UK, chaired the session in which the majority of the audience felt that the TASC III was attempting to consider too many eventualities.
The discussion highlighted the difficulties that TASC III contributors will have in achieving a workable consensus. To the question “Did you expect TASC anatomical lesions to change from 2000 to 2006?” a majority of voters, 71%, said “yes”. To “How should TASC III lesions be classified?” 1) Fixed anatomically, so that treatment modality changes over time? 68% voted yes. Fifty nine per cent of the audience then said this would NOT determine to which discipline a patient is referred. Then, 73% voted that the individual clinician should determine mode of intervention”. The vote to the last question was particularly telling, 75% voted yes to the question: “Is there a danger of TASC III attempting to consider too many eventualities?”
Balancing consensus and evidence
A theme that recurred was the issue of balancing consensus and scientific evidence. Greenhalgh requested a comment on how consensus needs to take into account evidence and in what way. To this, Lammer replied: “With TASC IIb, the challenge really was the lack of evidence. The question is, and this is a problem also for TASC III, how much should consensus overrule evidence, or lack of evidence?” In the session, William Hiatt, Denver, USA, who spoke on the topic “Why was TASC initiated and what is achievable?”, said the goal of TASC I was to achieve a consensus in the “management of individual patients with identical conditions.” TASC I aimed to represent all relevant key disciplines… and indentify and represent minority views. It was begun in 1996 and published in 2000,” he said. Hiatt noted that TASC I was the first major vascular guideline that represented all vascular disciplines across Europe and North America and achieved consensus on all but one recommendation. He said that it established TASC lesion classification to guide revascularisation decisions. Hiatt noted that the TASC III Writing Group consisted of Chapter Groups with a lead author and chapter Writing Group members who represent a diversity of expertise, vascular disciplines, societies, and geography.
“Every reference is reviewed prospectively for quality and relevance. The writing group had declared all industry disclosures and there was appropriate separation from industry support,” he noted. Hiatt also told CX delegates that the disagreements on specific recommendations were noted in final text with societies in agreement and in disagreement listed. He also outlined what was achievable with TASC III at CX 34. “TASC III seeks to achieve a consensus between surgical and endovascular societies. The response to TASC IIb was disappointing but current inter-societal engagement is encouraging,” he noted. On the question of whether it was possible to create a more integrated and clinically-relevant classification of peripheral arterial disease, he noted that TASC III would include the key components of: patient, limb and lesions. He added that TASC III would establish new standards for regulatory approval and reimbursement of new therapies. For critical limb ischaemia, it would expand on amputation-free survival to include healing of ischaemic ulcers, relief of ischaemic pain, avoidance of additional procedures and improvement in functional status, he said. Lars Norgren, Örebro/Lund, Sweden, then spoke on “The purpose of TASC II and TASC IIb; What is expected of TASC III?” He said that while TASC (2000) was intended mainly for specialists, TASC II aimed also at reaching referring physicians focussing on:
Key aspects on diagnosis and management
Updating and providing new information
Making graded recommendations
The resulting TASC classification was related to the appropriate mode of revascularisation with TASC A lesions being appropriately treated by endovascular means, D appropriate for open surgery, endovascular revacularisation being preferred for TASC B and surgical revascularisation for C. “The outcome of TASC II was >1600 citations on Google Scholar of which some were critical. Therefore TASC IIb set out to update the TASC lesion classification; add an infrapopliteal classification and clarify the role of endovascular treatment (vs. surgery) based on technical developments and professional skill despite the lack of level 1 evidence,” said Norgren. He told delegates that the principal conclusion of TASC IIb was an endovascular first approach, with open surgery for complex lesions or endovascular failure. “However, surgical societies saw this conclusion as being weighted too much in favour of endovascular therapies and the recommendations for open surgery as too weak,” he said. He also outlined some of the controversial issues pertaining to TASC IIb. “Scientifically valid rigorous guidelines are expected, but the trials rarely exist. The recommendations were based on grade C evidence in line with practical handling. In TASC, TASC II, TASC IIb, the recommendations were based on single lesion (anatomical) management,” he said. “Consensus was not achieved despite endorsement from most societies, and the decision was made to move to TASC III,” Norgren added. He told delegates that in preparation of TASC III, “All basic information achieved for TASC IIb is set to be further discussed and included as appropriate. There is also a move to expand discussion from prior TASC guidelines, which were lesion-based to a patient/limb/lesion consideration. The point that critical limb ischaemia ≠ intermittent claudication was being highlighted and TASC III would consider issues of experience, availability, resources. All strategies (including endovascular/surgery and hybrid) require active and honest individual and societal discussion for consensus,” he said.
What is expected of TASC III?
Norgren told delegates that TASC III was expected to satisfy the need for all specialists, comply with technical development, update the evidence grading, modify the change in evidence, focus on relevant classification(s), increase referencing, reflect the situation in developing countries and stay truly global. “Currently, all societies are committed, relevant Chapter Groups have been formed, there is an ongoing literature search and details of evidence grading are being worked out,” he said.
What were the problems with TASC IIb?
Michael R Jaff, Boston, Massachusetts, said that TASC was a true attempt to collaborate across nations and specialties to develop guidelines for the diagnosis and management of peripheral arterial disease.
“So, why TASC IIb?,” he asked, and then outlined that significant time had passed since the publication of TASC II. There has been a rapid expansion of endovascular technologies to treat peripheral arterial disease and major practice shifts to an “endovascular first” paradigm. He said that the goal of IIb was to update TASC II as an interim report and summarise published literature since TASC II. There was also a goal to update the anatomic recommendations regarding treatment strategies and outline situations where an “endovascular first” approach is reasonable. Jaff noted that Vascular Surgery had been unwilling to sign off on TASC IIb, claiming that the recommendations were based on low quality literature. “The Society for Vascular Surgery could not endorse an ‘endovascular first’ approach for any anatomic scenario,” Jaff noted.
He said that vascular surgeons would identify and emphasise the weakness of catheter-based intervention, poor durability in infrainguinal peripheral arterial disease and say that surgical patency rates were far superior. They would also say that catheter-based intervention could burn bridges to surgery down the road, was costly and the data was limited and of poor quality. “So, why endovascular first?” he asked and outlined that it was a low-risk procedures in skilled hands that was unlikely to “burn” the surgical bridges. Also, “Surgical revascularisation is not without risk, cost, and need for repeat intervention, similar to percutaneous transluminal angioplasty. The ideal surgical candidate is becoming harder to find due to older age, tissue loss and poor conduit. The ideal management will likely be integrated/hybrid on a background of comprehensive medical therapy.” In a talk titled “Scientific evidence or expert opinion?”
Jim Reekers, Amsterdam, The Netherlands, emphasised that evidence-based guidlines should be based on scientific evidence. “Expert opinion can be used to comment on, or interpret scientific evidence, and in the absence of evidence, can be used as the opinion of an expert or someone who knows a lot about a topic. However, expert opinion is not scientific evidence and should only be used when scientific evidence is lacking or cannot be generalised for a whole population,” he said. Reekers made the point that TASC III would be a scientific document if it was evidence-based, and a political document if it was a consensus document which was based on expert opinion.
Henrik Sillesen, Copenhagen, Denmark, noted that one major change from TASC I to TASC II was how indications for revascularisation were put into clinical perspective, not least how important risk factor management and proper medical treatment should be advocated as the primary treatment in non limb-threatening peripheral arterial disease (claudication). Sillesen noted that indication for invasive treatment of patients with lower limb vascular disease depends on severity of symptoms in the context of the patient’s history, function and situation in general. It also depends on results of non-invasive treatment, location and extent of lesion(s), and multiple segments (aorto-iliac, femoral and crural). In short, he said, it depends on the individual patient. On the other hand, Sillesen noted, the TASC classification of lesions, which is based on degree of stenosis on angiograms, does not predict symptoms, does not predict ankle brachial index and does not predict outcome. Images of arteries (angiograms) does not reflect function “Guidelines for treatment should reflect indication, options, including the non-invasive option, and evidence before recommending anatomy-based invasive treatment,” said Sillesen. Therefore, he said, the TASC classification should only be used as a surrogate for actually describing the lesions.
Sillesen made the point that guidelines were valued by “those who write them, scientific societies and those who happen to have a practice that fits to the guidelines.Many are unhappy with guidelines as they feel it limits their options and some feel that guidelines are unnecessary because they, after all, are doctors and can think for themselves,” he said. Then, Vincent Riambau, Barcelona, Spain, told delegates that intermittent claudication and ischaemic rest pain are included in TASC (2000 and 2007) recommendations and advices. “There is an absence of meaningful Grade A data comparing surgical to endovascular strategies, revascularisation recommendations are mainly Grade C and evolving technology is not enough to justify continuous modifications on strategy management. TASC III will need more evidence in terms of comparative revascularisation strategies and cost-effective analysis,” he said. Erich Minar, Vienna, Austria, made the point that he did not believe that anatomical treatment recommendations were of any use in clinical practice. He said that the simplified classification of TASC A, B, C, and D did not really have the same importance as clinical presentation (ie claudication vs. critical limb ischaemia, lifestyle limitation, comorbidities and age). Minar noted that there is poor inter-observer agreement on the TASC II classification of femoropopliteal lesions, citing T Kukkonen et al; Eur J Vasc Endovasc Surg 2010 and showed the image of a lesion that correlated poorly with none of the TASC II classes. Minar said that the “C” in TASC stood for compromise, rather than consensus. “Compromise is a settlement of differences in which each side makes concessions. Is this really in the interest of each patient ? Is a political compromise in the interest of each person?“ he questioned.
Live from CX 34: Slim majority do not believe drug elution is worthwhile
cxsymposium2015-07-11T02:23:24+01:00In an extremely close vote, 51% of delegates voted against the motion that drug elution is worthwhile for the management of occlusion of the infrainguinal arteries (vs. 49% who voted for the motion).
Supporting the motion were Thomas Zeller (Bad Krozingen, Germany) and Gunnar Tepe (Rosenheim, Germany) and against the motion were Stephan Duda (Berlin, Germany) and Iris Baugmartner (Bern, Switzerland).
Live from CX 34: TASC III – What is achievable?
cxsymposium2015-07-11T02:23:24+01:00William R Hiatt, Denver, USA, outlined what was achievable with TASC III at CX 34. He said TASC III was seeking to achieve a consensus between surgical and endovascular societies. “The response to TASC IIb was disappointing but current inter-societal engagement encouraging,” he noted.
On the question of whether it was possible to create a more integrated and clinically-relevant classification of peripheral arterial disease, he noted that TASC III would include the key components of: patient, limb and lesions.
He noted that TASC III would establish new standards for regulatory approval and reimbursement of new therapies. For critical limb ischaemia, it would expand on amputation-free survival to include healing of ischaemic ulcers, relief of ischaemic pain, avoidance of additional procedures and improvement in functional status, he said.
Live from CX 34: Endovascular techniques must be taught with interdisciplinary collaboration, 55% of CX delegates say
cxsymposium2020-01-08T13:52:57+00:00In a close vote, most delegates attending the first debate of CX 34 agreed with Sigrid Nikol, Hamburg, Germany, that endovascular techniques must be taught with interdisciplinary collaboration, with 55% supporting the motion vs. 45% who voted against the motion (and thus agreed with Frank Vermassen, Ghent, Belgium).
Nikol argued that the “excellent” results of endovascular therapy in clinical trials indicate that: “cardiologists, surgeons, radiologists, and other interventionists possessing peripheral vascular interventional skills can perform endovascular therapy safely despite different starting points.” On the other hand, Vermassen argued that training in endovascular techniques can and should be performed in vascular surgery training centres, saying: “Interdisciplinary collaboration is not a prerequisite for training in endovascular techniques.”
Controversies back in the limelight at CX34
cxsymposium2020-01-08T13:52:03+00:00The Charing Cross International Symposium (London, UK, 14–17 April 2012) returns this year beginning another three-year cycle of themes: Controversies, Challenges and Consensus. This year’s CX promises high-profile debates between leading vascular and endovascular specialists. The programme also includes several educational opportunities, latest clinical trial data and industry-sponsored symposia.
The Vascular News Charing Cross Special Edition highlights some of the controversial topics which will take centrestage at CX34.
The future of TASC
The first Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC) was published in 2000 to provide an international consensus on the diagnosis and treatment of peripheral arterial disease. With the aim of continuing to reach a readership of vascular specialists, but also physicians in primary healthcare, TASC II was published in 2007. TASC IIb was intended to be a technical update set to elaborate on the role of endovascular techniques in relation to surgery, and to include guidance on more severe lesions than those covered in TASC II, but was never published.
Many physicians are interested either in understanding why TASC IIb did not emerge after much discussion between disciplines and key societies, or listening to issues being discussed now as TASC III approaches. There are various questions to address: Will anatomical classification stay fixed and treatments then be seen to be becoming more endovascular each time? Why was TASC initiated and what is it possible to achieve with the classification? What were the aims of TASC IIb and what can be expected of TASC III?
Roger Greenhalgh, chairman, CX Programme and editor-in-chief of Vascular News said: “Some key authors of TASC III will speak and the audience will better understand the issues, have their say and even vote on issues. This will be a type of huge, open, focus group. This will be in no way official, nor threatening, but the discussion could be very helpful for TASC III authors. If anatomy is to change repeatedly to fit mode of treatment, is this a turf war in disguise?”
Aneurysm screening
The prevalence of abdominal aortic aneurysm and aneurysm rupture has decreased in recent years as a result of reduced exposure to risk factors, mainly smoking. How is this affecting aneurysm screening programmes? In a session on aortic screening, CX delegates will hear about screening experiences from the UK, Sweden and Australia and their progress.
Which TEVAR technique?
One of the most pressing topics in thoracic intervention is whether the varying types of pathologies require specific devices. “A common view is that some endovascular devices are more suitable for some pathologies than others,” Greenhalgh said.
In a debate, Peter Taylor, London, UK, will try to convince delegates that thoracic aortic pathology is key to choice of treatment. Dittmar Boeckler, Heidelberg, Germany, will attempt to convince the audience to stick to a favourite device whatever the pathology.
Faculty members will also try to answer which technique is the first choice for each pathology, including total surgical repair; total endovascular repair with branched grafts, with off-the-shelf pre-loaded fenestrated grafts and with physician-made fenestrated grafts; hybrid repair; sandwich repair; and chimney repair.
Multilayer stent
Is the multilayer stent (MARS) approach a breakthrough or not? Michel Henry, Nancy, France, Charles McCollum, Manchester, UK, and Thomas Larzon, Orebro, Sweden, will present their experiences with the device. A discussion involving the panel and delegates will take place after the talks. “We need to hear hard outcome data on this to either take up this approach, or not. We approach it with an open mind,” Greenhalgh said.
Is CCSVI an entity?
This year, a session will be dedicated to discussions around chronic cerebrospinal venous insufficiency on Tuesday 17 April. Three years ago, Paolo Zamboni sparked controversy with the presentation of results indicating that patients with multiple sclerosis benefited from endovenous treatment.
“Chronic cerebrospinal venous insufficiency is very controversial. It is not yet clear if it is an entity or not or how often internal jugular venous stenosis is found without symptoms. If it is an entity, then correction of the venous stenosis provides a possibility of improving the condition in many patients,” Greenhalgh said.
“Is chronic cerebrospinal venous insufficiency a real phenomenon? We all need to know the answer to this because if it is, patients will benefit. Doctors and patients need to know as soon as possible either to call for more of these procedures or to stop doing them altogether,” he added.
The Great Debate “CCSVI: It is an entity and a subset of multiple sclerosis” will see Zamboni and Manish Mehta speaking for the motion, and Alun Davies and Richard Nicholas arguing against the motion. After the debate, the four presentations will focus on the main questions about CCSVI, followed by a discussion with the audience.
CX34 delegates will also see several debates on other topics in vascular and endovascular intervention such as drug-elution, the CREST trial and venous disease.
Study investigates whether a SWIFT transport system improves outcomes of ruptured aneurysm repair
cxsymposium2020-01-08T13:51:14+00:00A clinical study involving all vascular centres in Switzerland treating leaking aneurysms will try to answer whether the time from diagnosis to intervention is related to operative death from ruptured abdominal aortic aneurysms.
The details of the SWIFT (Swiss ruptured aneurysm favourable transport) study were presented at the CX@LINC symposium in Leipzig in January by Regula von Allmen, Vascular Research Group, Imperial College London, UK.
In 2010, according to the Swiss National Vascular Registry, which included data from 21 of 25 Swiss centres, there were 109 ruptured aneurysm repairs and 611 elective aneurysm repairs in Switzerland. In the registry report, 30-day mortality for ruptured aneurysm was 23.8% in Switzerland. In the UK, for example, the Hospital Episode Statistics show operative mortality rates up to 46% for ruptured abdominal aortic aneurysms, Von Allmen noted. “Why is there an interest in Switzerland? Some excellent results have been reported in this country. Switzerland is a circumscribed country and the centres performing vascular surgery are clearly identified,” she said.
She told delegates that there are great variations between centres in the country. “Reports from Zurich show that 50.2% of the ruptured aneurysm patients are managed by endovascular means and 49.8% by open repair. Zurich reports excellent results for EVAR with a 30-day mortality rate of 13.5%. Open repair has a mortality rate of 32.4%. But if patients look anatomically unsuitable for EVAR, then this may not be a fair comparison between open and endovascular repair. However, when we look at data from Bern, there are only 4% who are treated by endovascular means and the vast majority, 96%, is treated with open repair, and the overall 30-day mortality is 15.3%.
“From this we can see that there are pockets of excellence in the treatment of ruptured abdominal aortic aneurysm in Switzerland. As a consequence, it is claimed by one centre that it is unethical to carry out treatment other than EVAR for ruptured aneurysms, but there are obvious counterclaims for open repair based upon the data from Bern. However, the point is we cannot assume that the surgical method alone holds the key. That is why we feel that there is a need for the SWIFT study, including all the Swiss vascular centres,” she added.
SWIFT will be a prospective, all inclusive, study to be conducted for two years. The hypothesis is that the time from first clinical diagnosis of suspected rupture to the time of beginning the intervention relates to 30-day procedural mortality.
The primary endpoint is 30-day surgical mortality; however, rates for death during transfer and turn down rates with reasons for this will also be reported. Key additional factors are the condition of the patient on arrival at hospital, anatomical features assessed by CT scans, and type of transport.
“So why are Swiss results so good? Is it surgical skills? Is it the method? Is it patient selection? Could it relate to swift transfer?” Von Allmen questioned. “Being a circumscribed country, Switzerland has excellent transport facilities with good roads and helicopter use. For instance, cardiologists have proved that results of treatment of acute coronary episodes improved when the time from diagnosis to catheterisation was reduced, the so-called door-to-balloon time. Can it be the same for ruptured abdominal aortic aneurysms? With the participation of all 25 Swiss centres, we may be able to answer this question.”
Von Allmen will give the talk "The time from diagnosis to the operating suite:SWIFT" at the Charing Cross Symposium on 15 April, London, UK.
St George’s Valve Technology course now part of the CX Symposium
cxsymposium2020-01-08T13:50:36+00:00For the first time, the St George’s Valve Technology Symposium—now in its seventh year—will be an integral part of the Charing Cross International Symposium. Now called the CX St George’s Valve Technology Symposium, the event will take place during the first two days of the 34th CX Symposium (14–17 April 2012, Imperial College London, UK). Its theme, in keeping with the CX Symposium’s this year’s overall theme of “Controversies”, is “Valve Technology Controversies” in percutaneous valve techniques.
The first day of the symposium (Saturday 14 April) will focus on managing thoracic aorta diseases and will include talks on preventing paraplegia in aortic surgery and the endovascular management of acute type B dissection. The second day (Sunday 15 April) will review the management of valvular heart disease. The programme complements the thoracic day (Monday 16 April) of CX.
Marjan Jahangiri, professor of Cardiac Surgery, St George’s Hospital, University of London, UK, and programme director of the CX St George’s Valve Technology Symposium, told Cardiovascular News that there would also be a series of debates and case presentations during the two-day event. She added: “As the techniques are now more effective, this is a very good opportunity to discuss controversies around new modalities for the treatment of valve disease.”
The main discussion will happen around the topics surgery vs. transcatheter aortic valve implantation, minimally invasive surgery vs. transcatheter approach and the different techniques of percutaneous intervention. Mitral and tricuspid valve intervention will also be addressed.
According to Jahangiri, the programme is aimed at interventional cardiologists, cardiac and cardiothoracic surgeons, cardiac theatre and cath lab nurses and technicians, cardiac anaesthestists, and intensive care specialists. She explained that there would be opportunities for all delegates, especially trainees, to try some of the new technologies on mannequins.
Each year, the CX Symposium attracts more than 3,000 delegates from 75 countries. For more information on registering to attend the symposium, which is Europe’s longest running vascular symposium, and the CX St George’s Valve Technology Symposium, visit: www.cxsymposium.com
Mid-term results for endovenous ablation support shift to office-based vein practice
cxsymposium2020-01-08T13:49:31+00:00Setting up an office-based vein practice is becoming extremely popular as the treatment of varicose veins is undergoing a revolution. Many physicians feel that it is becoming less and less acceptable for varicose veins to be operated on by using open surgical techniques.
CX heralds era of “off-the-shelf” fenestrated grafts
cxsymposium2020-01-08T13:47:50+00:00Largest hands-on office-based vein practice course in the world at CX
cxsymposium2020-01-14T09:59:16+00:00For the fourth year running, the Charing Cross Office Based Veins Course offered a vast selection of the latest therapies to treat varicose veins in an office environment. Over two sessions, 300 participants were trained on steam thermotherapy, mechano-chemo ablation, foam sclerotherapy, thread vein treatment and endovenous ablation, among other techniques.
CX News: Are the EVAR boundaries being possibly pushed too far?
cxsymposium2020-01-08T13:45:34+00:00Clinical practice must change today, according to the latest data from the 10-year follow-up of the UK EVAR trials and a large US M2S dataset registry. The anatomical suitability for EVAR must be respected and mandatory pre-discharge imaging should be a requirement in order to identify perioperative rupture, CX delegates heard yesterday. Remarkably, they also heard that the incidence of aortic sac enlargement after EVAR appears to be increasing, not decreasing, despite increased operator experience and improved device technology, and that this is due in part to the liberalisation of the anatomic characteristics deemed suitable for EVAR, and physicians expanding the indications for EVAR and using devices outside of the instructions for use (IFU)
Cluster of known endovascular complications
60% of EVAR cases do not meet the recommendations
The results of the study showed that in 10,228 patients undergoing EVAR during the study period, 59% had a maximum aneurysm diameter below the 5.5cm threshold where intervention is recommended over surveillance. Only 42% of patients had anatomic characteristics that met the most conservative definition of device instructions for use and 69% met the most liberal definition of device instructions for use.
Schanzer et al’s study had a mean follow up 31 ± 18 months, the mean number of follow up scans was 3.03 ± 0.9. The important findings that he discussed at CX were:
- Only 40% of aneurysms treated with EVAR meet the recommended diameter threshold for treatment (≥5.5 cm).
- Even applying the most liberal instructions for use definition, 30% of treated patients do not meet aortic neck criteria.
- Anatomy deemed acceptable for EVAR has continued to liberalise and several of these factors are independently associated with aortic aneurysm sac enlargement.
- Aortic sac enlargement post EVAR occurred in 40% of patients by five years.
- This incidence is significantly higher than that reported in industry-sponsored trials and reports from single centres of excellence.
- In >30% of patients demonstrating aortic sac enlargement, this was not detected until >3 years postoperatively.
- The incidence of aortic sac enlargement after EVAR seems to be increasing, not decreasing—despite increased surgeon experience and improved device technology.